Abstract
The principle of electrochromic modulation of excited-state intramolecular proton-transfer reaction was applied for the design of fluorescence probes with high two-color sensitivity to dipole potential, Ψd, in phospholipid bilayers. We report on the effect of Ψd variation on excitation and fluorescence spectra of two new 3-hydroxyflavone probes, which possess opposite orientations of the fluorescent moiety in the lipid bilayer. The dipole potential in the bilayer was modulated by the addition of 6-ketocholestanol or phloretin and by substitution of dimyristoyl phosphatidylcholine lipid with its ether analog 1,2-di-o-tetradecyl-sn-glycero-3-phosphocholine, and its value was estimated by the reference styryl dye 1-(3-sulfonatopropyl)-4-{β[2-(di-n-octylamino)-6-naphthyl]vinyl}pyridinium betaine. We demonstrate that after Ψd changes, the probe orienting in the bilayer similarly to the reference dye shows similar shifts in the excitation spectra, whereas the probe with the opposite orientation shows the opposite shifts. The new observation is that the response of 3-hydroxyflavone probes to Ψd in excitation spectra is accompanied by and quantitatively correlated with dramatic changes of relative intensities of the two well separated emission bands that belong to the initial normal and the product tautomer forms of the excited-state intramolecular proton-transfer reaction. This provides a strong response to Ψd by change in emission color.
Keywords: 3-hydroxyflavone dyes, two-color ratiometric probes
Some of the biological membrane properties are not easy to address and evaluate. One of them is the dipole potential, Ψd, and its dependence on the bilayer composition. Ψd is the potential formed between the highly hydrated lipid heads at the membrane surface and the low-polar interior of the bilayer (1, 2). It arises from the aligned dipolar residues of phospholipid molecules, with the participation of hydration water molecules on the level of their carbonyl and phosphate groups. Ψd produces a strong virtual positive charge in the bilayer center and is thought to be responsible for many membrane properties as, for instance, the substantial (up to 6 orders of magnitude) difference in the penetration rates between positively and negatively charged hydrophobic ions (3, 4). Meanwhile, the exact Ψd values, the role and quantitative contribution of each of its chemical determinants, and the distribution of Ψd across the bilayer are debated (5, 6). The estimates of the absolute Ψd values for phosphatidylcholine bilayers vary from –280 mV, as evaluated from different penetration rates of hydrophobic ions (4), to –500 mV, as computed from molecular dynamic simulation data (7). Direct measurements of this potential, which are possible only on phospholipid air–water or water–mercury monolayers (5, 6), support the Ψd existence but disagree quantitatively with the Ψd values obtained in bilayers (8, 9).
There are probably several sites for Ψd generation. Because of their orientation normal to the bilayer plane, the sn2-carbonyls are thought to be the primary determinants (1, 2, 4, 10), whereas the sn1-carbonyls oriented parallel to the bilayer plane are thought to provide only a small influence (Fig. 1). Other contributions to Ψd derive from the –P—N+ dipole (11) and from the P O bonds of phosphate groups (12). Additionally, the polarized dipoles of hydration water, which can be ordered and oriented perpendicular to the bilayer plane (7, 10, 13), may also contribute to Ψd generation. The presence of these highly polarized and hydrogen-bonded water molecules on the level of phosphate and even ester groups has been inferred by different methods (14, 15), but the connection between the dipole potential and bilayer hydration is still unclear.
O bonds of phosphate groups (12). Additionally, the polarized dipoles of hydration water, which can be ordered and oriented perpendicular to the bilayer plane (7, 10, 13), may also contribute to Ψd generation. The presence of these highly polarized and hydrogen-bonded water molecules on the level of phosphate and even ester groups has been inferred by different methods (14, 15), but the connection between the dipole potential and bilayer hydration is still unclear.
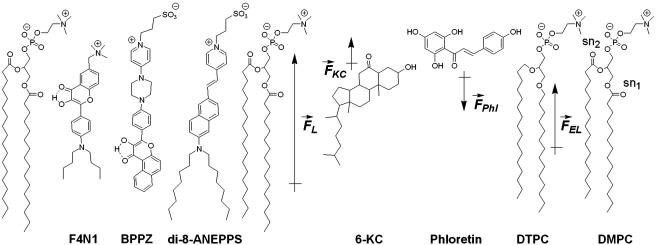
Fig. 1.
3HF probes F4N1 and BPPZ and their location in phosphatidylcholine bilayer leaflet. Styryl probe di-8-ANEPPS is presented for comparison. The orientations of F4N1 and BPPZ were derived from previous experiments (27) and analogies with similar probes (33). The lipids DMPC and DTPC [ether lipid (EL)] and lipid additives 6-KC and phloretin as well as their contribution to the membrane dipole potential (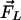 ,
, 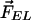 ,
, 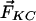 , and
, and 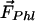 , respectively) are shown.
, respectively) are shown.
Moreover, the influence on Ψd of several factors modifying the properties of bilayer was described (Fig. 1). It was reported that phloretin, a compound with one carbonyl and four hydroxylic groups, strongly reduces the Ψd value (4, 16–18), whereas sterols including cholesterol increase it. One of the sterol compounds with a strong Ψd increasing effect is 6-ketocholestanol (6-KC) (4, 19). Furthermore, the removal of sn-carbonyls, which is realized in ether phospholipids, is an other mean to decrease Ψd (10).
Because direct Ψd measurement in the bilayer is not possible, a number of indirect methods have been suggested. The most universal, sensitive, and simple-in-performance method is based on fluorescence probes. Its application is not destructive and provides the possibility of sensing electrostatic effects at the molecular level. Moreover, its sensitivity allows the use of probe concentrations in the 10–6 to 10–8 M range, giving probe/lipid ratios ≤1:100. In this respect, styryl dyes, which were primarily designed as fast-responding probes for transmembrane potential (20), were later suggested as Ψd sensors (21–23). Their response is based on electrochromism (Stark effect), resulting in shifts of absorption and emission bands under the influence of an electric field (24). These dyes were designed in such a way that their charged group is anchored to the lipid heads, and the rod-like chromophore is oriented across the bilayer by attachment of hydrocarbon tails at the opposite side of the molecule. The basic mechanism of electrochromic charge transfer imposes some limits to the extent of probe response that could not be overcome by modification of the chromophore moiety. Therefore, to increase fluorescence sensitivity to Ψd, new probes responding by a different mechanism have to be designed.
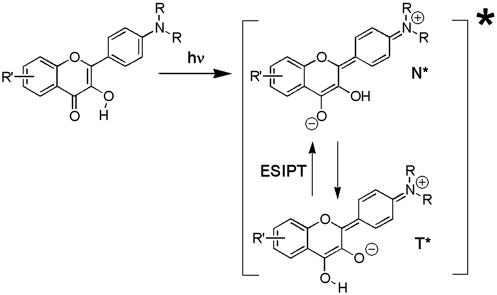
In this respect, an interesting observation was performed recently on charged 3-hydroxyflavone (3HF) derivatives (25). These molecules exhibit an excited-state intramolecular proton-transfer (ESIPT) reaction and consequently a dual fluorescence with two emission bands, corresponding to the initially excited normal (N*) and reaction product tautomer (T*) forms (26). The introduction of charges in proximity but not in direct conjugation with the chromophore not only shifts the absorption and emission bands in accordance with electrochromic mechanisms but also changes dramatically the intensity ratio of the N* and T* band in accordance with the spectral shifts (25). Thus, we describe a previously uncharacterized phenomenon, the electrochromic modulation of ESIPT reaction. This modulation has opened possibilities for convenient and sensitive observation of electrostatic field effects by recording the dramatic changes in emission color, which can hardly be achieved by the common electrochromic dyes. Recently we reported on newly synthesized membrane probes based on 3HF (27). These probes possess charged groups to anchor the chromophore to the membrane surface and hydrophobic substituents to orient them toward the low-polar part of the bilayer. In the present study, two members of this family with the 3HF moiety oriented in opposite directions with respect to the phospholipid bilayer plane are investigated. These probes were incorporated into phospholipid vesicles, and the dependence of their fluorescence properties on the dipole potential was investigated. The dipole potential was varied either by the addition of phloretin and 6-KC or by substitution of phospholipids with corresponding ether derivatives. The response of the probes was compared with that of the reference electrochromic dye 1-(3-sulfonatopropyl)-4-{β[2-(di-n-octylamino)-6-naphthyl]vinyl}pyridinium betaine (di-8-ANEPPS) (21). The results demonstrate that the probes operating by the ESIPT principle are prospective sensors for dipole potential in biomembranes.
Theory
The physical background of the response of 3HF dyes to applied electric fields is electrochromism (Stark effect) (25). It consists in the shifts of light absorption and emission bands caused by the interaction of an electric field with the ground-state and excited-state dipole moments of the chromophore (24). In the simplest dipole approximation that disregards electronic polarization effects, the direction and magnitude of the shift, Δνobs, is proportional to the electric field vector 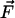 , and the change of dipole moment associated with the spectroscopic transition
, and the change of dipole moment associated with the spectroscopic transition 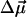 ,
,
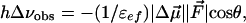 |
where θ is the angle between 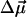 and
and 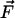 vectors, and εef is a microscopic analog of dielectric constant ε, which accounts for dielectric screening. Thus, to show maximal sensitivity to electrostatic potential, the probe should exhibit substantial change of its dipole moment
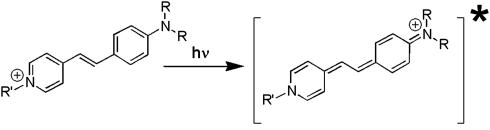
vectors, and εef is a microscopic analog of dielectric constant ε, which accounts for dielectric screening. Thus, to show maximal sensitivity to electrostatic potential, the probe should exhibit substantial change of its dipole moment 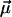 on electronic excitation, which implies a substantial redistribution of the electronic charge density. Furthermore, the probe should be located in low-polar environment (low εef) and oriented parallel (cosθ = 1) or antiparallel (cosθ = –1) to the electric field. Styryl dyes with electron-donor and electron-acceptor substituents at the opposite ends of the rodshaped conjugated π-electronic systems are among the best-known electrochromic dyes (20–23). They exhibit strong excited-state redistribution of electronic charge that can be modulated by electric field. Typical of this class of compounds are the 4-dialkylaminostyrylpyridinium dyes, which exhibit the excited-state reaction shown in Scheme 1. Importantly, 4′-dialkylamino-3HFs, which exhibit the ESIPT reaction shown in Scheme 2, are also characterized by a significant excited-state charge transfer in the N* state occurring from its 4′-dialkylamino to the 4-carbonyl group (28, 29). This charge transfer process is consistent with recent solvatochromic (30) and electrochromic (25, 31) data. Moreover, in the ESIPT transition from the normal N* to the tautomer T* excited state 4-oxygen acts as a proton acceptor to form the excited-state 4-hydroxyl. As a consequence, the resulting T* state shows a small electronic charge separation (i.e., dipole moment), which explains the weak solvatochromism (30) and electrochromism (25) of this state. Accordingly, an applied electric field may substantially change the energy of the N* state, resulting in common electrochromic shifts, but only marginally influence the T* state. Moreover, because ESIPT in 3HFs is a fast reversible two-state reaction, the electric field should influence the distribution of excited species between the N* and T* states and therefore the emission intensities of the corresponding bands. Thus, if an electric field is applied to the 3HF chromophore, the N* state, depending on the direction of the field, will either be stabilized or destabilized with respect to the T* state and its relative intensity will either be increased or decreased. This provides the strong coupling between electrochromism and ESIPT, which was established in our previous study (25). This coupling implies that the shifts in the excitation spectra and the N* emission band are always accompanied by changes in the relative intensities of the N* and T* bands. Because the N* and T* bands are strongly separated on the wavelength scale, the changes of their relative intensities provide a much stronger change in emission color than the band shifts.
on electronic excitation, which implies a substantial redistribution of the electronic charge density. Furthermore, the probe should be located in low-polar environment (low εef) and oriented parallel (cosθ = 1) or antiparallel (cosθ = –1) to the electric field. Styryl dyes with electron-donor and electron-acceptor substituents at the opposite ends of the rodshaped conjugated π-electronic systems are among the best-known electrochromic dyes (20–23). They exhibit strong excited-state redistribution of electronic charge that can be modulated by electric field. Typical of this class of compounds are the 4-dialkylaminostyrylpyridinium dyes, which exhibit the excited-state reaction shown in Scheme 1. Importantly, 4′-dialkylamino-3HFs, which exhibit the ESIPT reaction shown in Scheme 2, are also characterized by a significant excited-state charge transfer in the N* state occurring from its 4′-dialkylamino to the 4-carbonyl group (28, 29). This charge transfer process is consistent with recent solvatochromic (30) and electrochromic (25, 31) data. Moreover, in the ESIPT transition from the normal N* to the tautomer T* excited state 4-oxygen acts as a proton acceptor to form the excited-state 4-hydroxyl. As a consequence, the resulting T* state shows a small electronic charge separation (i.e., dipole moment), which explains the weak solvatochromism (30) and electrochromism (25) of this state. Accordingly, an applied electric field may substantially change the energy of the N* state, resulting in common electrochromic shifts, but only marginally influence the T* state. Moreover, because ESIPT in 3HFs is a fast reversible two-state reaction, the electric field should influence the distribution of excited species between the N* and T* states and therefore the emission intensities of the corresponding bands. Thus, if an electric field is applied to the 3HF chromophore, the N* state, depending on the direction of the field, will either be stabilized or destabilized with respect to the T* state and its relative intensity will either be increased or decreased. This provides the strong coupling between electrochromism and ESIPT, which was established in our previous study (25). This coupling implies that the shifts in the excitation spectra and the N* emission band are always accompanied by changes in the relative intensities of the N* and T* bands. Because the N* and T* bands are strongly separated on the wavelength scale, the changes of their relative intensities provide a much stronger change in emission color than the band shifts.
Scheme 1.
Excited-state charge transfer in styryl dyes.
Scheme 2.
ESIPT in 3HF dyes.
In biomembranes, the parallel orientation between 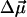 and the dipole potential gradient
and the dipole potential gradient 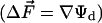 can be realized by achieving a vertical orientation of the chromophore to the plane of membrane. For optimal sensitivity to Ψd, the probe should be located at the level of maximal electric field gradient, probably on the level of the phospholipid carbonyl groups. The penetration depth can be controlled by the attached charged groups that interact with the lipid heads. This principle is realized in the most popular styryl dyes, di-8-ANEPPS (20, 21) and RH 421 (22, 23). In contrast to styryl dyes, the positively charged anchor in 3HF derivatives is not a part of the chromophore, and therefore the connecting spacer may be of variable length. In addition, it seems to be possible to design probes with opposite orientations with respect to the bilayer plane. Their comparative studies may exclude spectroscopic effects of factors that do not depend on probe orientation with respect to the electric field vector such as polarity or viscosity.
can be realized by achieving a vertical orientation of the chromophore to the plane of membrane. For optimal sensitivity to Ψd, the probe should be located at the level of maximal electric field gradient, probably on the level of the phospholipid carbonyl groups. The penetration depth can be controlled by the attached charged groups that interact with the lipid heads. This principle is realized in the most popular styryl dyes, di-8-ANEPPS (20, 21) and RH 421 (22, 23). In contrast to styryl dyes, the positively charged anchor in 3HF derivatives is not a part of the chromophore, and therefore the connecting spacer may be of variable length. In addition, it seems to be possible to design probes with opposite orientations with respect to the bilayer plane. Their comparative studies may exclude spectroscopic effects of factors that do not depend on probe orientation with respect to the electric field vector such as polarity or viscosity.
Materials and Methods
Absorption spectra were measured on Cary 3 Bio and Cary 400 (Varian) spectrophotometers. Fluorescence spectra were recorded on an SLM 48000 (SLM–Aminco, Urbana, IL) spectrofluorometer. Phloretin, 6-KC, pluronic F-127, and egg yolk phosphatidylcholine (EYPC) were purchased from Sigma and used without further purification. Dimyristoyl phosphatidylcholine (DMPC) and 1,2-di-o-tetradecyl-sn-glycero-3-phosphocholine (DTPC) were from Avanti Polar Lipids.
The probe di-8-ANEPPS was purchased from Molecular Probes. The synthesis of N-[(4′-dimethylamino)-3-hydroxy-6-flavonyl]methyl-N,N-trimethyl ammonium bromide (F4N1) has been described in detail (27). 3-(4-{4-[4′-(3-Hydroxybenzo[f] flavonyl)phenyl]piperazino}-1-pyridiniumyl)-1-propanesulfonate (BPPZ) was synthesized starting from 2-hydroxy-1-acetylnaphthalene and 4-[4-(4-pyridyl)piperazino]benzaldehyde by using the procedure described for its parent analogue 4-{4-[4′-(3-hydroxyf lavonyl)]piperazino}-1-(3-sulfopropyl)pyridinium (PPZ) (27). Melting point > 280°C (decomposition); UV max in acetonitrile 383 nm, ε = 32,000 liters·mol–1 per cm–1; 1H NMR (300 MHz, DMSO-D6) 2.09 (2H, m), 2.37 (2H, t, J = 7.0 Hz), 3.58 (4H, m), 3.91 (4H, m), 4.31 (2H, t, J = 6.7 Hz), 7.15 (2H, d, J = 8.1 Hz), 7.30 (2H, d, J = 6.8 Hz), 7.69 (1H, t, J = 8.4 Hz), 7.81 (1H, t, J = 8.4 Hz), 7.87 (1H, d, J = 9.1 Hz), 8.23 (2H, d, J = 8.1 Hz), 8.3 (1H, d, J = 9.1 Hz), 8.38 (2H, d, J = 6.8 Hz), 9.40 (1H, s), 10.01 (1H, d, J = 8.4 Hz); mass spectrum (fast atom bombardment) m/z 571.2 (M+).
Experiments were performed systematically with large unilamellar vesicles obtained as described (32). The final lipid concentration in all the experiments was 200 μM in 15 mM Hepes buffer, pH 7.4. Probes were added to lipid vesicles, under stirring, at 1% ratio (mol/mol) from millimolar stock solutions in DMSO for 3HF probes and in methanol for di-8-ANEPPS. Phloretin and 6-KC were incorporated into large unilamellar vesicles from millimolar stock solutions in DMSO + 2.5% Pluronic F-127 according to the procedure described by Gross et al. (21).
Results
The present research is based on application of the recently reported membrane 3HF probe F4N1 (27) and the newly synthesized BPPZ. Fig. 1 represents the structures of these probes together with their suggested location and orientation with respect to the phospholipid bilayer as determined from previous studies (27) by the parallax method and comparison with other membrane probes (33). The probe F4N1 contains a small positively charged trimethylammonium group that can interact electrostatically with the lipid phosphate groups. Being anchored to the bilayer surface by this group, F4N1 extends its low-polar flavone moiety into the hydrophobic part of the bilayer. The large hydrophobic 4′-dibutylamino substituent favors an orientation of the chromophore orthogonal to the bilayer plane. The BPPZ probe is a close analogue of the previously described probe PPZ (27). These probes are designed to orient oppositely in the membrane with respect to the probe F4N1. The additional benzene ring in the BPPZ molecule (with respect to PPZ) increases its hydrophobicity and screens its 4-carbonyl from hydrogen-bonding interaction with a protic environment (34). This may increase the affinity of the probe for the membrane, decrease hydration of the flavone moiety, and provide its deeper location and more vertical orientation in the bilayer.
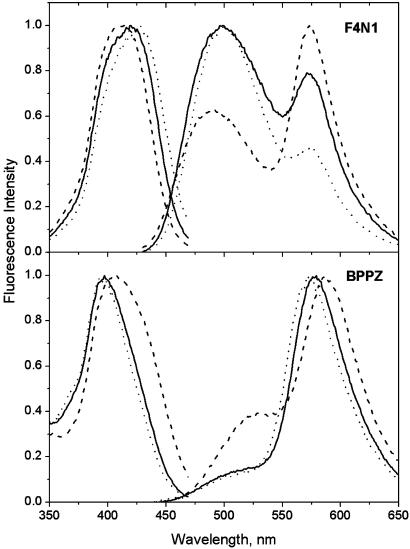
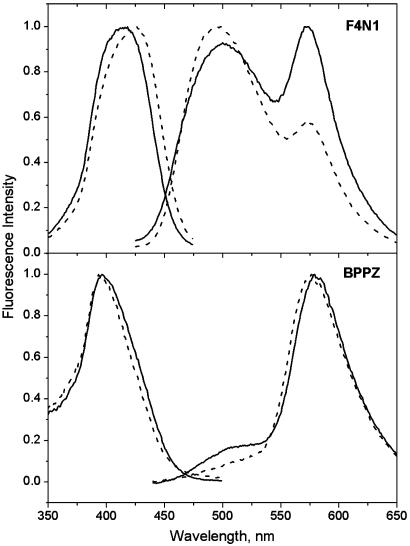
The excitation and fluorescence spectra of the probes F4N1 and BPPZ in EYPC vesicles are illustrated in Fig. 2. For the probe F4N1, the excitation spectrum is represented by a single band, the position of which (at 421 nm) does not depend significantly on emission wavelength. The fluorescence spectrum consists of two strongly Stokes-shifted emission bands; one at the shorter wavelengths (500 nm) belongs to the N* form, and the other at the longer wavelengths (573 nm) belongs to the T* form. The presence of these two bands in comparable intensities is characteristic for different 3HF derivatives in phospholipid membranes (27, 35, 36). The excitation spectrum of BPPZ in EYPC vesicles is blue-shifted with respect to that of F4N1 with a maximum at 397 nm, which is similar to that observed in ethanol. The position of the maximum is also almost independent of the emission wavelength. The fluorescence spectrum is represented by emission bands of the N* form with a maximum around 510 nm (appears as a shoulder) and of the T* form at 578 nm. The relative intensity of the N* band with respect to the T* band (IN*/IT*) is significantly lower than that observed in the vesicles for the probe PPZ, (analog of BPPZ) which shows already a deep location in the bilayer (27). Moreover, the absolute value of the IN*/IT* ratio in the studied vesicles corresponds to that for 4′-dialkylamino-3HFs in apolar solvents (30, 34). These results suggest that the chromophore moiety of the probe BPPZ is deeply imbedded into the low-polar central part of the bilayer.
Fig. 2.
Excitation and emission spectra of probes F4N1 and BPPZ in EYPC vesicles in the absence (solid lines) or presence of dipole potential modifiers: 50% 6-KC (dashes) or 20% phloretin (dots). Temperature, 20°C. Emission spectra were measured at an excitation wavelength of 400 nm. Excitation spectra were recorded at the T* band maximum.
The dipole potential in EYPC vesicles can be modulated by the addition of phloretin and 6-KC, which decreases and increases its value, respectively (refs. 1, 2, 4, and 16–18 and Fig. 1). We observe that with the addition of 50% of 6-KC, the excitation spectrum of the probe F4N1 shifts by 7 nm (400 cm–1) to the shorter wavelengths. A similar blue shift (8 nm, 330 cm–1) is observed for the N* band emission, whereas almost no shift is detected for the T* band emission. These shifts are accompanied by a 2-fold decrease in the IN*/IT* ratio (Fig. 2). As expected, all the spectroscopic effects in the presence of phloretin were opposite (the excitation spectrum and the emission bands shift to the red, and the IN*/IT* ratio increases) to those observed with 6-KC, in line with the property of phloretin to decrease Ψd. The sign and magnitude of the observed shifts in excitation spectra with the addition of phloretin and 6-KC are similar to those observed for di-8-ANEPPS (21).
In principle, the addition of phloretin and 6-KC may change not only Ψd but also the polarity, viscosity, and hydration of the probe-binding site (16–19). Moreover, these additives also may interact with the probe directly. Because the effect of the vectorial parameter, Ψd, should depend strongly on probe orientation, in contrast to the other mentioned factors that are scalar and thus orientation-independent, it is important to study in the same conditions the probe BPPZ with opposite chromophore orientation in the bilayer. As expected, the spectroscopic effects observed with this probe on the addition of 6-KC and phloretin to EYPC vesicles are the opposite of those with the probe F4N1. The addition of 6-KC results in the red shifts of the excitation and emission spectra and also in a strong increase of the IN*/IT* ratio. The spectroscopic effects are opposite in sign but of similar amplitude to those observed with the probe F4N1. Meantime, the opposite effects of phloretin on BBPZ (blue shift of the emission spectra and decrease of the IN*/IT* ratio) are significantly smaller than those observed with the probe F4N1. These results suggest that the studied probes respond to the addition of 6-KC mainly due to the vectorial electrochromic effect, whereas the response to phloretin may also contain a contribution due to changes of the membrane properties and its direct interaction with the probes.
The discrepancy in the response of the two probes to phloretin prompted us to apply another approach to decrease the dipole potential in the bilayer: the substitution of the phospholipids with the corresponding ether analogs (ref. 10 and Fig. 1). According to our data (Fig. 3), the probe F4N1 demonstrates significant red shift of the excitation spectrum (10 nm, 560 cm–1) obtained with vesicles composed of the ether lipid DTPC with respect to that obtained with DMPC vesicles. The same effect is observed with the reference probe di-8-ANEPPS. The shift in the case of F4N1 is accompanied by an ≈2-fold increase of the relative intensity of the N* band. This effect is observed for vesicles in both the gel (14°C) (data not shown) and liquid-crystalline (38°C) (Fig. 3) phases. As expected, the response of the probe BPPZ to the lipid substitution is the opposite. Although the shift in excitation again is smaller than with F4N1, the value of the IN*/IT* ratio is decreased almost 2-fold.
Fig. 3.
Excitation and emission spectra of probes F4N1 and BPPZ in DMPC (solid lines) and DTPC (dashes) vesicles. Temperature, 38°C. Excitation and emission wavelengths are the same as described for Fig. 2.
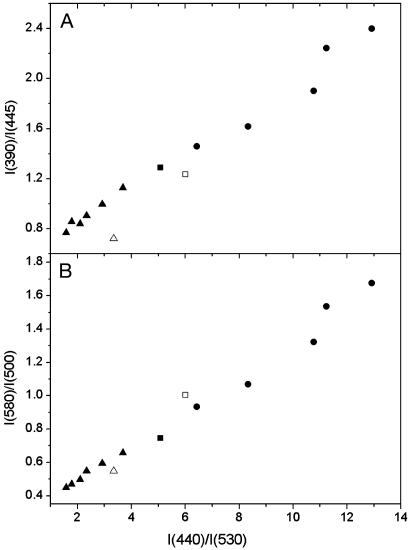
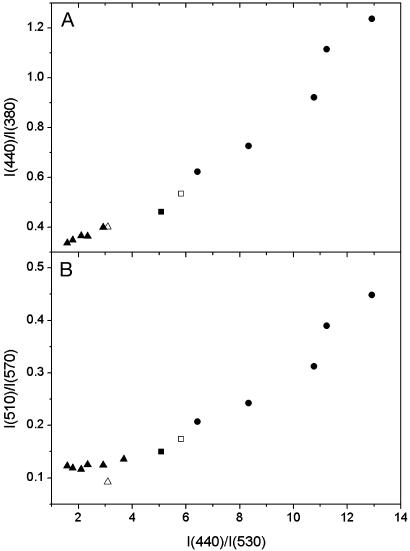
In view of the uncertainty in the absolute Ψd magnitude, its scaling in the present work is provided by the reference electrochromic probe di-8-ANEPPS. Commonly, the dipole potential changes are inferred from the shifts of excitation spectrum of this probe by measuring the intensity ratio at the edges of the spectrum, I440/I530 (21). To validate further the use of our probes for measuring the Ψd changes, we investigated the quantitative correlation between the di-8-ANEPPS response and the responses of our probes in the same experimental conditions. The shifts of the excitation bands of our probes can be evaluated in the same way as for di-8-ANEPPS. Thus, we chose for F4N1 the corresponding I390/I445 ratio. Because the response for BPPZ is observed in the opposite direction, the reversed ratio was used, I440/I380. The correlations of these ratios with di-8-ANEPPS data are satisfactory for both F4N1 (Fig. 4A) and BPPZ (Fig. 5A).
Fig. 4.
Correlation of the ratiometric response of probe F4N1 to the dipole potential with that of di-8-ANEPPS. Intensity ratios for F4N1 at the excitation spectrum edges, I390/I445 (A), and at the N* and T* maxima of emission spectrum, I580/I500 (B), are represented as a function of the excitation ratio I440/I530 for di-8-ANEPPS. The dipole potential is modified in EYPC vesicles (▪) by the addition of phloretin (▴) or 6-KC (•) at 20°C and also by the substitution of DMPC (□) by DTPC (▵) at 38°C. Di-8-ANEPPS excitation spectra were recorded at an emission wavelength of 645 nm.
Fig. 5.
Correlation of the ratiometric response of probe BPPZ to the dipole potential with that of di-8-ANEPPS. Intensity ratios for BPPZ at the edges of the excitation spectrum, I440/I380 (A), and at the maxima of the emission spectrum, I510/I570 (B), are represented as a function of the excitation ratio I440/I530 for di-8-ANEPPS. Experimental conditions and symbols are the same as described for Fig. 4. Note that, because the response of BPPZ is opposite to that of F4N1, the corresponding ratios were inverted.
To analyze further the correlation with the ratiometric response at the N* and T* band maxima of emission spectra, we chose the ratios I580/I500 for F4N1 and I510/I570 for BPPZ. For F4N1, the linear correlation with the response of di-8-ANEPPS holds both in the case of Ψd modifiers and for the DMPC–DTPC substitution (Fig. 4B). In the case of BPPZ, a good correlation is observed for the experiments with 6-KC and DMPC–DTPC, whereas the corresponding points with phloretin show some deviation (Fig. 5B), which is probably due to the side effects of phloretin mentioned above.
Thus, we demonstrate that the response of the probes F4N1 and BPPZ both in excitation and emission spectra show quantitative correlation with the response in excitation of the reference probe di-8-ANEPPS for all types of experiments that modify Ψd. This provides strong evidence for the electrochromic mechanism of the response of our probes and makes them applicable for quantitative measurements of Ψd changes.
Discussion
The major idea in the background of this research is the development of a strategy in the design of fluorescence probes highly sensitive to membrane electrostatic potentials. This idea is realized successfully with ESIPT reaction in newly synthesized 3HF derivatives. Previously we demonstrated that a proximal charged group can dramatically modulate ESIPT reaction in 3HF, resulting in strong changes in the relative intensities of the two emission bands (25). In the present report we show that 3HF probes F4N1 and BPPZ inserted vertically in the lipid bilayer are highly sensitive to its dipole potential. Thus, increase in Ψd destabilizes the N* state of the probe F4N1, because its dipole orients against the field similarly to that of di-8-ANEPPS (Fig. 1). This results in a blue shift of the excitation and the N* emission bands. Meantime, the T* state with much smaller dipole moment (30) is not affected. The relative destabilization of the N* state shifts the ESIPT equilibrium toward the T* state, leading to a dramatic decrease of the IN*/IT* ratio. In contrast, the N* state of BPPZ with an increase of Ψd is stabilized, which results in the opposite spectral effects. Importantly, the relatively small shifts in excitation and emission spectra are coupled with strong variations of intensities of two well separated emission bands that result in amplification of the color-changing response.
We have to stress the unique properties of 3HFs as fluorescence probes. Because four distinct electronic states (N and T ground states and N* and T* excited states) and N* ↔ T* reaction are involved in their fluorescence response, the different characteristics of the microenvironment (polarity, hydrogen bonding, and electric field effects) can be described by a number of independently measured spectroscopic variables such as the position of absorption (or excitation) and two emission band maxima and the IN*/IT* ratio (30). Our results show that for the previously uncharacterized probes the excitation spectrum always shifts in accordance with the changes in the dipole potential, whereas in the case of the N* band there are several exceptions (Figs. 2 and 3). The abnormal behavior of the N* band is connected with its strong solvatochromism (30) such that the excited-state relaxation effects could compensate for the electrochromic effects. This is probably one of the reasons why the probe di-8-ANEPPS shows lower electrochromic response in emission spectra compared with that in excitation spectra. Application of the highly electrochromic ESIPT reaction (25) for the newly synthesized dyes allowed the achievement of a strong ratiometric response to Ψd in emission, which was not possible before with the application of probes operating by the charge-shift mechanism. Moreover, these probes allow recording changes in relative intensity of two well resolved separate bands, which is far more convenient and precise for two-color detection in spectroscopy and microscopy of the living cell than the recording of the ratiometric effects on the narrow regions of the band edges. Finally, the previously uncharacterized probes are prototypes of two-band fluorescent ratiometric probes for electric fields in lipid membranes that operate on ESIPT principle, and various possibilities are foreseen for improving their spectroscopic and sensing properties (37, 38).
Acknowledgments
This work was supported in part by TUBITAK Research Institute for Genetic Engineering and Biotechnology and Centre National de la Recherche Scientifique (CNRS). A.P.D. was a fellow from the French Ministère de la Recherche and Collège Doctoral Européen. A.S.K. is presently a fellow from the CNRS.
Abbreviations: 6-KC, 6-ketocholestanol; 3HF, 3-hydroxyflavone; ESIPT, excited-state intramolecular proton transfer; di-8-ANEPPS, 1-(3-sulfonatopropyl)-4-{β[2-(di-n-octylamino)-6-naphthyl]vinyl}pyridinium betaine; EYPC, egg yolk phosphatidylcholine; DMPC, dimyristoyl phosphatidylcholine; DTPC, 1,2-di-o-tetradecyl-sn-glycero-3-phosphocholine; F4N1, N-[(4′-dimethylamino)-3-hydroxy-6-flavonyl]methyl-N,N-trimethyl ammonium bromide; BPPZ, 3-(4-{4-[4′-(3-Hydroxybenzo[f]flavonyl)phenyl]piperazino}-1-pyridiniumyl)-1-propanesulfonate; PPZ, 4-{4-[4′-(3-hydroxyflavonyl)]piperazino}-1-(3-sulfopropyl)pyridinium.
References
- 1.Brockman, H. (1994) Chem. Phys. Lipids 73, 57–79. [DOI] [PubMed] [Google Scholar]
- 2.Clarke, R. J. (2001) Adv. Colloid Interface Sci. 89–90, 263–281. [DOI] [PubMed] [Google Scholar]
- 3.Liberman, Y. A. & Topaly, V. P. (1969) Biofizika 14, 452–455. [PubMed] [Google Scholar]
- 4.Franklin, J. C. & Cafiso, D. S. (1993) Biophys. J. 65, 289–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moncelli, M. R., Becucci, L., Tadini Buoninsegni, F. & Guidelli, R. (1998) Biophys. J. 74, 2388–2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Belucci, M., Moncelli, M. R., Herrero, R. & Guidelli, R. (2000) Langmuir 16, 7694–7700. [Google Scholar]
- 7.Mashl, R. J., Scott, H. L., Subramaniam, S. & Jakobsson, E. (2001) Biophys. J. 81, 3005–3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Herrero, R., Moncelli, M. R., Guidelli, R., Carla, M., Arcangeli, A. & Olivotto, M. (2000) Biochim. Biophys. Acta 1466, 278–288. [DOI] [PubMed] [Google Scholar]
- 9.Peterson, U., Mannock, D. A., Lewis, R. N. A. H., Pohl, P., McElhaney, R. N. & Pohl, E. E. (2002) Chem. Phys. Lipids 117, 19–27. [DOI] [PubMed] [Google Scholar]
- 10.Gawrisch, K., Ruston, D., Zimmerberg, J., Parsegian, V. A., Rand, R. P. & Fuller, N. (1992) Biophys. J. 61, 1213–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bechinger, B. & Seelig, J. (1991) Biochemistry 30, 3923–3929. [DOI] [PubMed] [Google Scholar]
- 12.Diaz, S., Lairión, F., Arroyo, J., Biondi de Lopez, A. C. & Disalvo, E. A. (2001) Langmuir 17, 852–825. [Google Scholar]
- 13.Marrink, S.-J., Tieleman, D. P., van Buuren, A. R. & Berendsen, H. J. C. (1996) Faraday Discuss. 103, 191–201. [Google Scholar]
- 14.Wiener, M. C., Suter, S. M. & Nagle, J. F. (1989) Biophys. J. 55, 315–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hübner, W. & Blume, A. (1998) Chem. Phys. Lipids 96, 99–123. [Google Scholar]
- 16.Cseh, R. & Benz, R. (1999) Biophys. J. 77, 1477–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cseh, R., Hetzer, M., Wolf, K., Kraus, J., Bringmann, G. & Benz, R. (2000) Eur. Biophys. J. 29, 172–183. [DOI] [PubMed] [Google Scholar]
- 18.Jendrasiak, G. L., Smith, R. L. & McIntosh, T. J. (1997) Biochim. Biophys. Acta 1329, 159–168. [DOI] [PubMed] [Google Scholar]
- 19.Simon, S. A., McIntosh, T. J., Magid, A. D. & Needham, D. (1992) Biophys. J. 61, 786–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Loew, L. M. (1982) J. Biochem. Biophys. Methods 6, 243–260. [DOI] [PubMed] [Google Scholar]
- 21.Gross, E., Bedlack, R. S., Jr., & Loew, L. M. (1994) Biophys. J. 67, 208–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clarke, R. J., Zouni, A. & Holzwarth, J. F. (1995) Biophys. J. 68, 1406–1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clarke, R. J. (1997) Biochim. Biophys. Acta 1327, 269–278. [DOI] [PubMed] [Google Scholar]
- 24.Bublitz, G. U. & Boxer, S. G. (1997) Annu. Rev. Phys. Chem. 48, 213–242. [DOI] [PubMed] [Google Scholar]
- 25.Klymchenko, A. S. & Demchenko, A. P. (2002) J. Am. Chem. Soc. 124, 12372–12379. [DOI] [PubMed] [Google Scholar]
- 26.Sengupta, P. K. & Kasha, M. (1979) Chem. Phys. Lett. 68, 382–385. [Google Scholar]
- 27.Klymchenko, A. S., Duportail, G., Ozturk, T., Pivovarenko, V. G., Mély, Y. & Demchenko, A. P. (2002) Chem. Biol. 9, 1199–1208. [DOI] [PubMed] [Google Scholar]
- 28.Chou, P.-T., Martinez, M. L. & Clements, J.-H. (1993) J. Phys. Chem. 97, 2618–2622. [Google Scholar]
- 29.Ormson, S. M., Brown, R. G., Vollmer, F. & Rettig, W. (1994) J. Photochem. Photobiol. A 81, 65–72. [Google Scholar]
- 30.Klymchenko, A. S. & Demchenko, A. P. (2003) Phys. Chem. Chem. Phys. 5, 461–468. [Google Scholar]
- 31.Nemkovich, N. A., Baumann, W. & Pivovarenko, V. G. (2002) J. Photochem. Photobiol. A 153, 19–24. [Google Scholar]
- 32.Lleres, D., Dauty, E., Behr, J.-P., Mély, Y. & Duportail, G. (2001) Chem. Phys. Lipids 111, 59–71. [DOI] [PubMed] [Google Scholar]
- 33.Kachel, K., Asuncion-Punzalan, E. & London, E. (1998) Biochim. Biophys. Acta 1374, 63–76. [DOI] [PubMed] [Google Scholar]
- 34.Klymchenko, A. S., Pivovarenko, V. G. & Demchenko, A. P. (2003) J. Phys. Chem. A 107, 4211–4216. [Google Scholar]
- 35.Bondar, O. P., Pivovarenko, V. G. & Rowe, E. S. (1998) Biochim. Biophys. Acta 1369, 119–130. [DOI] [PubMed] [Google Scholar]
- 36.Duportail, G., Klymchenko, A. S., Mely, Y. & Demchenko, A. P. (2001) FEBS Lett. 508, 196–200. [DOI] [PubMed] [Google Scholar]
- 37.Klymchenko, A. S., Ozturk, T., Pivovarenko, V. G. & Demchenko, A. P. (2001) Tetrahedron Lett. 42, 7967–7970. [Google Scholar]
- 38.Klymchenko, A. S., Ozturk, T., Pivovarenko, V. G. & Demchenko, A. P. (2002) Tetrahedron Lett. 43, 7079–7082. [Google Scholar]