Abstract
The 2002 discovery of a robust modern human mandible in the Peştera cu Oase, southwestern Romania, provides evidence of early modern humans in the lower Danubian Corridor. Directly accelerator mass spectrometry radiocarbon (14C)-dated to 34,000–36,000 14C years B.P., the Oase 1 mandible is the oldest definite early modern human specimen in Europe and provides perspectives on the emergence and evolution of early modern humans in the northwestern Old World. The moderately long Oase 1 mandible exhibits a prominent tuber symphyseos and overall proportions that place it close to earlier Upper Paleolithic European specimens. Its symmetrical mandibular incisure, medially placed condyle, small superior medial pterygoid tubercle, mesial mental foramen, and narrow corpus place it closer to early modern humans among Late Pleistocene humans. However, its cross-sectional symphyseal orientation is intermediate between late archaic and early modern humans, the ramus is exceptionally wide, and the molars become progressively larger distally with exceptionally large third molars. The molar crowns lack derived Neandertal features but are otherwise morphologically undiagnostic. However, it has unilateral mandibular foramen lingular bridging, an apparently derived Neandertal feature. It therefore presents a mosaic of archaic, early modern human and possibly Neandertal morphological features, emphasizing both the complex population dynamics of modern human dispersal into Europe and the subsequent morphological evolution of European early modern humans.
It has become apparent that the Late Pleistocene emergence of modern human biology in the peninsular northwestern Old World (Europe) was the complex result of the earlier emergence of modern humans in some portion of Africa, their subsequent dispersal over tens of millennia throughout Africa and Eurasia, and the geographically and temporally variable blending of those dispersing early modern human populations with regional groups of late archaic humans. This general scenario is supported by the Late Pleistocene human paleontological record (1–7) and extant human molecular data (8–10). Late Pleistocene mtDNA is compatible with this interpretation (11–14) and unlikely to refute it (15). As a consequence, the emphasis in the analysis of modern human emergence in peripheral regions such as Europe is shifting from a debate of polarized positions to more detailed considerations of the regional and temporal nuances of the evolutionary process.
In Europe, data have been accumulating concerning the biology of the latest Neandertals, dating to between 29 and 40 thousand years (ka) B.P. (2, 16–19), but there remains a dearth of well dated and morphologically diagnostic early modern human remains before ≈28 ka B.P. (20, 21). The only candidates for diagnostic early modern Europeans older than ≈28 ka B.P. are from Kent's Cavern (United Kingdom), Mladeč (Czech Republic), La Quina (France), Les Rois (France), and Vogelherd (Germany); all derive from old excavations, and only Kent's Cavern 4 is directly dated. The recent juvenescence of multiple purported early modern Europeans (2, 21–23) argues for caution. In the context of this limited knowledge of the biology of the earliest modern Europeans, it is difficult to address the more subtle aspects of the evolutionary emergence of those populations and their subsequent evolution. Moreover, well provenienced and diagnostic early modern human remains from the lower Danubian corridor are absent (24, 25). The recently discovered Peştera cu Oase in southwestern Romania and the Oase 1 human mandible help to fill some of this gap.
The Peştera cu Oase, the Oase 1 Mandible, and 14C Dating
The Peştera cu Oase (cave with bones) is a karstic chamber in the southwestern Carpathian Mountains, Romania. Discovered during speleological exploration by ş.M., A.B., and L.S., it contains multiple karstic geological formations, abundant large Late Pleistocene Ursus spelaeus, small carnivores, mammalian herbivores, and one mandible of Homo sapiens. The U. spelaeus remains appear to be from hibernation mortality, but the sources of the nonursid remains are currently unknown. Moreover, some of the ursid remains have been intentionally displaced within the cave in a pattern known from the Grotte Chauvet (26). The Oase 1 mandible was found on February 16, 2002 on the paleosurface near the current entrance to the chamber, and it too may have been moved in the past from its original location.
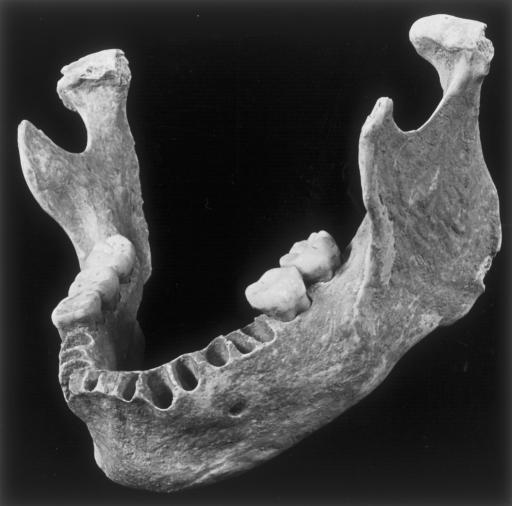
Oase 1 retains a complete corpus and left ramus, most of the right ramus, and five molars (Fig. 1).¶¶ There is no evidence of gnawing, and the only damage is marginal crushing and abrasion to the right posterior ramus and condylar margins. Because it was a paleosurface find within a karstic cavity, samples were removed from the inferior right ramus for direct accelerator mass spectrometry 14C dating.
Fig. 1.
Oblique view of the Oase 1 mandible.
Bone samples were prepared for accelerator mass spectrometry 14C dating at the Oxford Radiocarbon Accelerator Unit and the Centre for Isotope Research Radiocarbon Laboratory (Groningen, The Netherlands) by using routine collagen extraction procedures (27). An additional ultrafiltration pretreatment step was used at Oxford Radiocarbon Accelerator Unit to purify the bone gelatin and retain only the >30-kDa molecular mass fraction for dating (28). The lyophilized gelatin samples were combusted, mass spectrometrically analyzed, and then graphitized by reduction of CO2 over an Fe catalyst in an excess H2 atmosphere (29). We evaluated the quality of the dated collagen by using the atomic ratio of carbon to nitrogen (C:N), the percent weight collagen extracted from the bone, and the percent carbon after combustion as well as stable C and N isotopes (Table 1). C:N ratios should range between 2.9 and 3.6. Additional carbon atoms from a noncollagenous source will increase the C:N ratio and, depending on the age and size of the contaminant, may result in errors in the accelerator mass spectrometry results.
Table 1. Accelerator mass spectrometry 14C direct dating of the Oase 1 mandible.
| OxA-11711 | GrA-22810 | |
|---|---|---|
| Sample weight, mg | 350 | 706 |
| Organic (collagen) weight, mg | 1.5 | 28.5 |
| Collagen carbon content, % | 44.4 | 39.6 |
| δ13C, ‰ | -18.7 | -19.0 |
| C:N ratio | 3.3 | 2.6 |
| 14C activity ratio, % | 0.25 ± 0.5 | 1.40 ± 0.16 |
| 14C years B.P. | >35,200 | 34,290, +970, -870 |
OxA-11711 produced a yield of 4.3 mg/g, which is less than the usual minimum threshold (10 mg/g or 1% weight collagen). GrA-22810 produced a higher yield of 40.3 mg/g. We attribute this to the removal of low molecular mass (<30-kDa) components through the Oxford Radiocarbon Accelerator Unit ultrafiltration protocol, which retains larger peptides and excludes low molecular mass components, which can include salts, degraded and broken-up collagen, and sometimes material incorporated postdepositionally within the bone that can be of older, but usually younger, 14C age. The fact that both laboratories produced very similar ages suggests strongly that there is no difference in age between components in the bone that vary by molecular mass.
The errors in Table 1 are 1σ. The ages are in 14C years B.P. The 14C activity ratio (14a) is the ratio of the measured 14C activity of sample and reference, and it ranges between 0 and 1 (30). It is needed for a proper interpretation of “old” 14C ages and their error estimation and for the calculation of the 14C age T. For moderately old 14C ages, 14a ± σ(14a) easily translates into an age T ± σ(T); for old samples, the errors in T become asymmetric.
For measurements close to the 14C dating limit, the interpretation of the error term may become problematic. For OxA-11711 σ(14a) is >14a, such that the 1σ error range includes negative activities that correspond to infinite ages. For this reason, following convention, for cases where 14a < σ(14a) (31), the 14C activity for the measurement is taken as 14a + 2σ(14a), and the 14C age is calculated from this number and is considered the age limit. For OxA-11711, this result is a 14C age limit of 35,200 B.P.
When multiple measurements are undertaken, the mean result can be determined through averaging the activity ratios. For Oase 1, this provides a weighted average activity ratio of 〈14a〉 = 1.29 ± 0.15%, resulting in a combined OxA-GrA 14C age of 34,950, +990, and –890 B.P.
The 14C dating places Oase 1 contemporaneous with European late initial Upper Paleolithic and especially early Aurignacian archeological assemblages (32), even though no artifactual material has yet been identified in the Peştera cu Oase. It places it between the current dates of ≈31 ka B.P. for Kent's Cavern 4 (33), ≈32–33 ka B.P. for Vogelherd (34), and ≈34 ka B.P. for the Mladeč remains (23), on one hand, and the north African Nazlet Khater remains at ≈37 ka B.P. (35) on the other hand. It is older than late Neandertal specimens from the Crimea, Croatia, and Iberia (2, 18, 36–38), but it is long after the Middle Paleolithic oxygen isotope stage 5 early modern humans from southwestern Asia and east Africa (39). Oase 1 therefore falls between the earliest African [and by extension Near Eastern (41)] modern humans and those of the European early Upper Paleolithic (EUP), and it overlaps late surviving Neandertals. As such, it provides morphological data for the transition from Neandertals to early modern humans and a baseline for the subsequent evolution of “anatomically modern” Europeans.
The Morphology of Oase 1
The mandible plays a complex role in facial architecture, because it reflects the constraints of the nasopharyngeal complex, the neurocranial base, and the dental, muscular, and biomechanical demands of mastication. Few of the attributes of an isolated mandible are primary features of the facial anatomy, and most represent secondary consequences of more important aspects of facial biology. However, the morphological configurations of mandibles can be used to assess patterns of facial structure, bearing in mind the indirect natures of those reflections.
Assessments of Oase 1 are principally, with respect to its potential ancestral populations, the Neandertals, Near Eastern Middle Paleolithic early modern humans from Qafzeh and Skhul, and the penecontemporaneous early modern human from Nazlet Khater (42). Comparisons are also relevant to the EUP (>20 ka B.P.) European early modern humans.
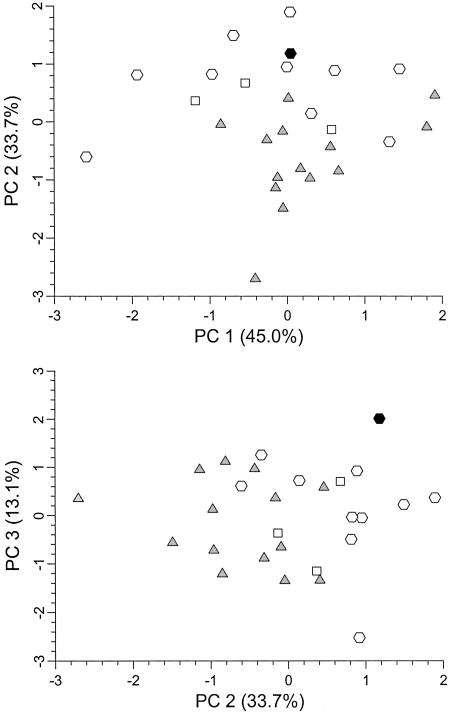
The overall size and proportions of the Oase 1 mandible align it most closely with EUP Europeans. A discriminant function analysis with size-adjusted (43) linear variables provides an 85.2% correct Neandertal vs. early modern human classification and places Oase 1 with the EUP sample with a posterior probability of 0.994. A principal components analysis of the same five variables indicates that, although it is only the second principal component that provides some degree of Neandertal–early modern human differentiation, Oase 1 is distinct from the Neandertals on axis 2 and distinct from all three samples on axis 3 (Fig. 2). The differences between Oase 1 and the Neandertals appear to be driven by its large ramus breadth and modest corpus breadth (Fig. 3 and Table 2). Among later Pleistocene human remains, only Nazlet Khater 2 has an absolutely wider ramus (51.0 mm). The ramus breadth to mandible length index of Oase 1 (44.7) is exceeded only by that of Nazlet Khater 2 (47.4) and approached by that of Pataud 1 (43.2) (Table 2). Similarly wide rami are otherwise known among Pleistocene Homo only for the Middle Pleistocene Arago 2, KNM-BK 67, Loyangalani 1, Mauer 1, and Tighenif 3 mandibles, as well as the later north African Dar-es-Soltane 5.
Fig. 2.
Bivariate plot of size-adjusted principal component (PC) scores for Late Pleistocene mandibular dimensions. Black hexagon, Oase 1; gray triangles, Neandertals; open squares, Qafzeh-Skhul early modern humans; open hexagons, EUP modern humans.
Fig. 3.
Oase 1 in norma lateralis left. The scale bar is in centimeters.
Table 2. Osteometrics of the Oase 1 mandible and comparative samples (in mm).
| Mandible superior length | Symphyseal height | Mental foramen height | Mental foramen breadth | Ramus minimum breadth | Ramus breadth-to-length index | |
|---|---|---|---|---|---|---|
| Oase 1 | 103.5 | 34.5 | 33.5/32.9 | 11.9/12.4 | 46.2 | 44.7 |
| Neandertals | 109.3 ± 6.7 (15) | 35.1 ± 3.8 (21) | 32.9 ± 3.3 (23) | 15.7 ± 1.8 (23) | 41.5 ± 2.7 (15) | 38.0 ± 2.4 (15) |
| Qafzeh-Skhul | 109.0, 118.0, 126.0 | 37.3 ± 3.2 (4) | 35.0, 36.0, 40.5 | 13.2, 15.0, 16.6 | 42.5, 43.0, 44.0 | 33.7, 37.3, 39.4 |
| EUP | 99.9 ± 7.3 (13) | 32.3 ± 3.6 (15) | 32.2 ± 4.1 (12) | 12.1 ± 1.4 (11) | 38.2 ± 3.4 (13) | 38.2 ± 3.0 (13) |
| P | <0.001 | 0.029 | 0.106 | <0.001 | 0.006 | 0.714 |
Mean ± SD (N) provided for sufficiently large comparative samples. Right/left provided for Oase 1 as preserved; right and left values are averaged for comparative specimens. P values are from ANOVA across the comparative samples.
The anterior symphysis of Oase 1 presents a prominent tuber symphyseos, but the lateral tubercles are minimally developed, providing it with a mentum osseum rank (44) of 4, the most common pattern among early modern humans (45, 46). Its anterior symphyseal angle (infradentale-pogonion/alveolar plane) falls between the Middle (Neandertal and Qafzeh-Skhul) and Upper Paleolithic samples, but its cross-sectional symphyseal angle (44) is in the overlap zone between Neandertal and early modern human mandibles (Table 3).
Table 3. Symphyseal angles relative to the alveolar plane for the Oase 1 mandible and comparative samples.
| Anterior symphyseal angle, ° | Cross-sectional symphyseal angle, ° | |
|---|---|---|
| Oase 1 | 91 | 84 |
| Neandertals | 80.8 ± 7.3 (18) | 75.7 ± 6.5 (18) |
| Qafzeh-Skhul | 89.3 ± 0.5 (4) | 85, 88, 91 |
| EUP | 96.3 ± 6.2 (12) | 93.3 ± 8.5 (10) |
| P | <0.001 | <0.001 |
Mean ± SD (N) provided for sufficiently large comparative samples. P values are from ANOVA across the comparative samples.
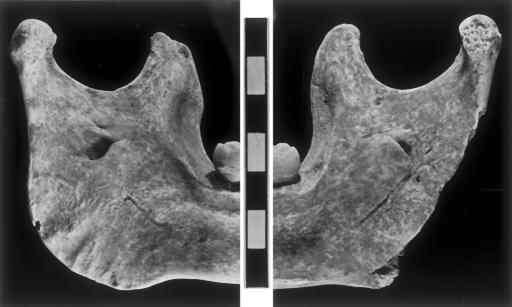
The discrete traits that differentiate Neandertal and early modern human mandibles in their frequency distributions (47) largely align Oase 1 with early modern humans. This applies to its mental foramina under each second premolar alveolus, retromolar space absence, symmetrical mandibular incisure, and lateral position of the incisure crest on the condyle (or medially placed condyle) (Figs. 3 and 4 and Table 4). However, all except the last feature are variable within the EUP sample, and at least one Neandertal (La Quina 9) presents the suite of early modern human features (47). The lingual bridging of the mandibular foramen, an apparently derived Neandertal lineage feature (49), is absent from the right ramus but present on the left one (Fig. 4); this feature is present in less than half of the Neandertal mandibles and appears occasionally in EUP specimens (Table 4).
Fig. 4.
Medial views of the Oase 1 mandibular rami. The scale bar is in centimeters.
Table 4. Discrete trait distributions for the Oase 1 mandible and comparative samples.
| Mental foramen, percent anterior of P4/M1 | Retromolar space, percent absent | Mandibular incisure, percent symmetrical | Incisure crest, percent lateral on condyle | Mandibular foramen, percent lingular bridging | |
|---|---|---|---|---|---|
| Oase 1 | P4 | Absent | Symmetrical | Lateral | Absent/present |
| Neandertals | 7.4 (27) | 25.0 (28) | 28.6 (14) | 62.5 (16) | 40.9 (22) |
| Qafzeh-Skhul | 66.7 (6) | 60.0 (5) | 100 (2) | 100 (2) | 0.0 (3) |
| EUP | 80.8 (26) | 77.1 (24) | 88.2 (17) | 100 (17) | 20.0 (15) |
| P | <0.001 | <0.001 | <0.001 | 0.005 | 0.069 |
P values are from Kruskal—Wallis tests across the comparative samples as exact probabilities (48). Sample sizes are provided in parentheses.
Of the preserved teeth (Fig. 5), only the third molars (M3s) provide extensive occlusal morphological data given the wear on the M1 and M2s. The two mesial molars appear to have had at least five cusps, and the M2s and M3s exhibit well developed hypoconulids [Arizona State University dental anthropology system (50) grade 3 or higher]. There are no midtrigonid crests on the M2s or M3s, and the M3s exhibit small (grade 1) entoconulids and modest anterior foveae. In addition, both P3 alveoli indicate that their roots possessed mesial developmental grooves (Tomes' root). All these features occur in varying frequencies among Late Pleistocene humans (51), although the absence of midtrigonid crests and the small dimensions of the anterior foveae suggest morphological affinities to early modern humans.
Fig. 5.

Occlusal view of the Oase 1 right mandibular molars from M1 to M3. The scale bar is in millimeters.
The dental dimensions of the Oase 1 molars are unusual. The Oase 1 molars become progressively larger from M1 to M3. With respect to buccolingual diameters, this pattern is relatively rare among Late Pleistocene humans [18.1% of EUP (n = 11) and Neandertal (n = 22) samples, 0.0% of Qafzeh-Skhul (n = 5)] and not significantly different across the samples (Kruskal–Wallis P = 0.146). Having an M3 larger than the M2 is more common among the Neandertals (46.2%, n = 26) than among early modern humans (EUP: 30.8%, n = 13; Qafzeh-Skhul: 0.0%, n = 7) and significantly different across the samples (Kruskal–Wallis P = 0.007).
Comparisons of M2 and M3 crown diameters (Table 5) indicates that, whereas the Oase 1 molar buccolingual diameters are large but well within Late Pleistocene human ranges of variation, the M2 and especially M3 mesiodistal diameters are exceptional (M1 interproximal attrition does not permit accurate determination of its length). The former are between 2.2 and 2.8 SD from the comparative means, and the latter are between 2.8 and 4.1 SD from the means (Table 5). Indeed, none of the Late Pleistocene specimens have M2 or M3 “areas” (length × breadth) that match those of Oase 1 (155.3 and 170.5 mm2, respectively). It is necessary to go to the late Middle Pleistocene (Krapina 53) to find a larger M2 (52) and to the earlier Middle Pleistocene (KNM-BK 8518) for a larger M3 (53). The Oase 1 M2 area is still above the mean of a pooled Old World Early and Middle Pleistocene archaic Homo sample (144.0 ± 25.2 mm2, n = 61). Its M3 area is 1.43 SDs above the mean of the earlier Pleistocene sample (134.9 ± 24.9 mm2, n = 46) and 2.42 SDs above the mean of a European Middle Pleistocene sample (123.0 ± 19.6 mm2, n = 26).
Table 5. Dental crown diameters of Oase 1 and Late Pleistocene comparative samples (in mm).
| M1 BL diameter | M2 MD diameter | M2 BL diameter | M3 MD diameter | M3 BL diameter | |
|---|---|---|---|---|---|
| Oase 1 | 11.7 | (12.9)/(13.2) | 12.1/11.7 | (14.2)/14.1 | 12.1/12.0 |
| Neandertals | 10.9 ± 0.6 (49) | 11.7 ± 0.5 (37) | 11.0 ± 0.7 (35) | 11.7 ± 0.6 (40) | 11.0 ± 0.8 (42) |
| Qafzeh-Skhul | 11.7 ± 0.6 (15) | 11.1 ± 0.8 (9) | 10.9 ± 0.7 (10) | 11.9 ± 0.8 (7) | 10.8 ± 0.7 (7) |
| EUP | 11.1 ± 0.7 (39) | 11.3 ± 0.8 (33) | 11.0 ± 0.7 (34) | 11.3 ± 0.8 (20) | 10.7 ± 0.8 (21) |
| P | 0.029 | 0.032 | 0.937 | 0.057 | 0.491 |
The Oase 1 mesiodistal (MD) values in parentheses are estimated values, correcting for interproximal wear. The preserved crown lengths are 12.6 and 12.8 mm for the M2s and 14.0 for the right M3. Mean ± SD (N) provided for sufficiently large comparative samples. P values are from ANOVA across the comparative samples. BL, buccolingual.
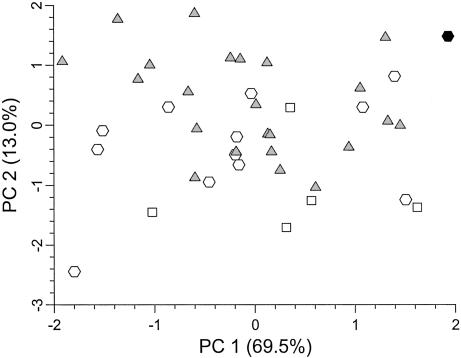
A Size- And shape-discriminant function analysis of these five dental diameters places Oase 1 with the Neandertals (posterior probability, 0.874), in which 82.5% of the specimens are classified correctly between late archaic and early modern humans. In a principal components analysis, a plot of the first two principal components (Fig. 6) shows that, on the first axis, Oase 1 is outside the Late Pleistocene distribution; on the second axis, it is among the Neandertals with the highest scores.
Fig. 6.
Bivariate plot of size and shape principal component (PC) scores for Late Pleistocene mandibular molar crown diameters. Black hexagon, Oase 1; gray triangles, Neandertals; open squares, Qafzeh-Skhul early modern humans; open hexagons, EUP modern humans.
Discussion
From these morphological comparisons, it is evident that the Oase 1 mandible presents a derived early modern human feature (the prominent tuber symphyseos) and aspects that place it closer to early modern humans among Late Pleistocene mandibles [overall proportions, more mesial mental foramen, narrow lateral corpus, retromolar space absence, symmetrical mandibular incisure, lateral incisure crest (or more medial condyle), and small superior medial pterygoid tubercle]. In a European oxygen isotope stage 3 context, these morphological patterns, and especially the tuber symphyseos, are sufficient to identify Oase 1 as an “early modern human.”
At the same time, Oase 1 presents an exceptionally wide ramus, both absolutely and relative to mandibular length. Because total mandible length is well within Late Pleistocene ranges of variation, falling between the means for the Middle and Upper Paleolithic samples (54) (Table 2), the ramal breadth indicates an unusually broad ramus and, by extension, a long temporal fossa and anterior positioning of the zygomatic bone. This pattern appears among African later archaic and early modern humans (55, 56); although present among Middle Pleistocene humans, it was variable among them.
The only feature that suggests Neandertal affinities is the lingual bridging of the mandibular foramen, a feature that is currently unknown among humans preceding Oase 1 other than the late Middle and Late Pleistocene members of the Neandertal lineage (49). It is present among European early modern humans (Table 3), but none of them is old enough to represent the ancestral lineage of Oase 1. The etiology of lingual bridging is poorly known, but its pattern of populational distribution suggests a strong genetic component (57). As previously argued (58), its presence in moderate frequencies among European early modern humans, now including Oase 1, implies some genetic contribution of the Neandertals to those subsequent populations.
The other unusual aspect of Oase 1 is its distal molar megadontia. The combination of large molar dimensions and the proportions along the tooth row can be considered archaic relative to early modern humans, because they align Oase 1 with both the Neandertals and preceding Middle and Early Pleistocene Homo. Their absence from the Middle Paleolithic Qafzeh-Skhul sample may be taken to suggest Neandertal affinities. However, two north African late Middle Pleistocene archaic humans, BOU-VP-16/1 and Irhoud 3, have at least M1 megadontia (7, 59) even though other and subsequent Late Pleistocene north Africans do not have particularly large molars (56, 60, 61).
The presence of archaic features in Oase 1, in the context of derived early modern human aspects, argues principally for significant craniofacial change within at least Europe after the establishment of modern humans across most of the region. Similar arguments have been made on the basis of dental dimensions (62), and the facial proportions of Aurignacian specimens such as Les Rois 1 and Mladeč 5 and 8 argue for similar changes (3, 40, 63). Given its earlier date and more pronounced archaic aspects, the Oase 1 mandible both reinforces and expands the extent to which “modern human” populations continued to evolve after their oxygen isotope stage 3 dispersal into portions of the Old World.
Conclusion
The 2002 discovery of a human mandible at the Peştera cu Oase in southwestern Romania indicates that the earliest “modern” Europeans combined a variety of archaic Homo, derived early modern human, and possibly Neandertal features in their craniofacial skeletal and dental morphology. Although compatible with some degree of admixture between regional Neandertal populations and in-dispersing early modern humans, the Oase 1 mandible is particularly relevant for emphasizing the degree to which early modern humans were not particularly modern.
Abbreviations: ka, thousand years; EUP, early Upper Paleolithic; Mn, nth lower molar.
Footnotes
The repository for Oase 1 is the Institutul de Speologie “Emil Racoviţă.” Casts are available through Mario Chech, Museé de l'Homme, Paris.
References
- 1.Holliday, T. W. (1997) J. Hum. Evol. 32, 423–447. [DOI] [PubMed] [Google Scholar]
- 2.Smith, F. H., Trinkaus, E., Pettitt, P. B., Karavanić, I. & Paunović, M. (1999) Proc. Natl. Acad. Sci. USA 96, 12281–12286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wolpoff, M. H., Hawks, J., Frayer, D. W. & Hunley, K. (2001) Science 291, 293–297. [DOI] [PubMed] [Google Scholar]
- 4.Bräuer, G. (2001) in Humanity from African Naissance to Coming Millennia, eds. Tobias, P. V., Raath, M. A., Moggi-Cecchi, J. & Doyle, G. A. (Witwatersrand Univ. Press, Johannesburg), pp. 183–189.
- 5.Stringer, C. (2002) Philos. Trans. R. Soc. London B 357, 563–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Trinkaus, E. & Zilhão, J. (2002) Trabalhos de Arqueologia 22, 497–518. [Google Scholar]
- 7.White, T. D., Asfaw, B., DeGusta, D., Gilbert, H., Richards, G. D., Suwa, G. & Howell, F. C. (2003) Nature 423, 742–747. [DOI] [PubMed] [Google Scholar]
- 8.Harding, R. M., Healy, E., Ray, A. J., Ellis, N. S., Flanagan, N., Todd, C., Dixon, C., Sajantila, A., Jackson, I. J., Birch-Machin, M. A., et al. (2000) Am. J. Hum. Genet. 66, 1351–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Relethford, J. H. (2001) Genetics and the Search for Modern Human Origins (Wiley–Liss, New York).
- 10.Templeton, A. R. (2002) Nature 416, 45–51. [DOI] [PubMed] [Google Scholar]
- 11.Nordborg, M. (1998) Am. J. Hum. Genet. 63, 1237–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tschentscher, F., Capelli, C, Geisert, H., Krainitzki, H., Schmitz, R. W. & Krings, M. (2000) in Neanderthals and Modern Humans: Discussing the Transition, eds. Orschiedt, J. & Weniger, G. C. (Neanderthal Museum, Mettmann, Germany), pp. 303–314.
- 13.Gutiérrez, G., Sánchez, D. & Marín, A. (2002) Mol. Biol. Evol. 19, 1359–1366. [DOI] [PubMed] [Google Scholar]
- 14.Caramelli, D., Lalueza-Fox, C., Vernesi, C., Lari, M., Casoli, A., Mallegni, F., Chiarelli, B., Dupanloup, I., Bertranpetit, J., Barbujani, G., et al. (2003) Proc. Natl. Acad. Sci. USA 100, 6593–6597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wall, J. D. (2000) Genetics 154, 1271–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vandermeersch, B. (1984) Bull. Mém. Soc. Anthropol. Paris Sér. XIV 1, 191–196. [Google Scholar]
- 17.Trinkaus, E., Churchill, S. E., Ruff, C. B. & Vandermeersch, B. (1999) J. Archaeol. Sci. 26, 753–773. [Google Scholar]
- 18.Hublin, J. J., Barroso Ruiz, C., Medina Lara, P., Fontugne, M. & Reyss, J. L. (1995) C. R. Acad. Sci. 321, 931–937. [Google Scholar]
- 19.Schmitz, R. W., Serre, D., Bonani, G., Feine, S., Hillgruber, F., Krainitzki, H., Pääbo, S. & Smith, F. H. (2002) Proc. Natl. Acad. Sci. USA 99, 13342–13347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Churchill, S. E. & Smith, F. H. (2000) Yearb. Phys. Anthropol. 43, 61–115. [Google Scholar]
- 21.Henry-Gambier, D. (2003) Bull. Mém. Soc. Anthropol. Paris 14, 89–112. [Google Scholar]
- 22.Terberger, T., Street, M. & Bräuer, G. (2001) Archäol. Korrespondenzblatt 31, 521–526. [Google Scholar]
- 23.Svoboda, J., van der Plicht, J. & Kuželka, V. (2002) Antiquity 76, 957–962. [Google Scholar]
- 24.Gleń, E. & Kaczawski, K. (1982) in Excavation in the Bacho Kiro Cave (Bulgaria), ed. Kozłowski, J. K. (Państwowe Wydawnictwo Naukowe, Warsaw), pp. 75–79.
- 25.Cârciumaru, M. (1999) Le Paléolithique en Roumanie (Millon, Grenoble, France).
- 26.Robert-Lamblin, J. (2001) in La Grotte Chauvet: L'Art des Origines, ed. Clottes, J. (Seuil, Paris), pp. 200–209.
- 27.Bronk Ramsey, C., Pettitt, P. B., Hedges, R. E. M., Hodgins, G. W. L. & Owen, D. C. (2000) Archaeometry 42, 459–479. [Google Scholar]
- 28.Brown, T. A., Nelson, D. E., Vogel, J. S. & Southon, J. R. (1988) Radiocarbon 30, 171–177. [Google Scholar]
- 29.Bronk Ramsey, C. & Hedges, R. E. M. (1997) Nucl. Instrum. Methods Phys. Res. 123B, 539–545. [Google Scholar]
- 30.Mook, W. G. & van der Plicht, J. (1999) Radiocarbon 41, 227–239. [Google Scholar]
- 31.Olsson, I. U. (1989) PACT Publications, 24, 161–177. [Google Scholar]
- 32.Svoboda, J., Havlček, P., Ložek, V., Macoun, J., Musil, R., Přichystal, A., Svobodová, H. & Vlček, E. (2002) Paleolit Moravy a Slezska (Archeologický ústav AV ČR, Brno, Czech Republic), 2nd Ed.
- 33.Stringer, C. B. (1990) in Hominid Remains: An Update. British Isles and Eastern Germany, ed. Orban, R. (Univ. Libre de Bruxelles, Brussels), pp. 1–40.
- 34.Conard, N. J. & Bolus, M. (2003) J. Hum. Evol. 44, 331–371. [DOI] [PubMed] [Google Scholar]
- 35.Vermeersch, P. M. (2002) in Palaeolithic Quarrying Sites in Upper and Middle Egypt, ed. Vermeersch, P. M. (Leuven Univ. Press, Leuven, Belgium), pp. 273–282.
- 36.Zilhão, J. (2000) in Neanderthals on the Edge, eds. Stringer, C. B., Barton, R. N. E. & Finlayson, J. C. (Oxbow, Oxford), pp. 111–121.
- 37.Walker, M. J. (2001) in A Very Remote Period Indeed, eds. Milliken, S. & Cook, J. (Oxbow, Oxford), pp. 153–159.
- 38.Marks, A. E. & Chabai, V. (2003) in Transitions Before the Transition, eds. Kuhn, S. & Hovers, E. (Plenum, New York), in press.
- 39.Valladas, H., Mercier, N., Joron, J. L. & Reyss, J. L. (1998) in Neandertals and Modern Humans in Western Asia, eds. Akazawa, T., Aoki, K. & Bar-Yosef, O. (Plenum, New York), pp. 69–75.
- 40.Smith, F. H. & Trinkaus, E. (1991) in Aux Origines d'Homo sapiens, eds. Hublin, J. J. & Tillier, A. M. (Presses Universitaires de France, Paris), pp. 251–290.
- 41.Holliday, T. W. (2000) Am. Anthropol. 102, 54–68. [Google Scholar]
- 42.Thoma, A. (1984) J. Hum. Evol. 13, 287–296. [Google Scholar]
- 43.Darroch, J. N. & Mosimann, J. E. (1985) Biometrika 72, 241–252. [Google Scholar]
- 44.Dobson, S. D. & Trinkaus, E. (2002) J. Hum. Evol. 43, 67–87. [DOI] [PubMed] [Google Scholar]
- 45.Teschler-Nicola, M. & Trinkaus, E. (2001) J. Hum. Evol. 40, 451–465. [DOI] [PubMed] [Google Scholar]
- 46.Trinkaus, E. (2002) Trabalhos de Arqueologia 22, 312–325. [Google Scholar]
- 47.Stefan, V. H. & Trinkaus, E. (1998) Bull. Mém. Soc. Anthropol. Paris 10, 293–324. [Google Scholar]
- 48.Mehta, C. & Patel, N. (1999) STATXACT for Windows (Cytel Software, Cambridge, MA), Version 4.
- 49.Lebel, S. & Trinkaus, E. (2002) J. Hum. Evol. 43, 659–685. [DOI] [PubMed] [Google Scholar]
- 50.Turner, C. G., II, Nichol, C. R. & Scott, G. R. (1991) in Advances in Dental Anthropology, eds. Kelley, M. & Larsen, C. (Wiley–Liss, New York), pp. 13–31.
- 51.Bailey, S. E. (2002) Ph.D. thesis (Arizona State Univ., Tempe).
- 52.Wolpoff, M. H. (1979) Am. J. Phys. Anthropol. 50, 67–114. [DOI] [PubMed] [Google Scholar]
- 53.Wood, B. A. & Van Noten, F. L. (1986) Am. J. Phys. Anthropol. 69, 117–127. [DOI] [PubMed] [Google Scholar]
- 54.Trinkaus, E. (2003) Proc. Natl. Acad. Sci. USA 100, 8142–8145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pinhasi, R. (2002) in Palaeolithic Quarrying Sites in Upper and Middle Egypt, ed. Vermeersch, P. M. (Leuven Univ. Press, Leuven, Belgium), pp. 283–335.
- 56.Ferembach, D. (1976) Bull. Mem. Soc. Anthropol. Paris Ser. 13 3, 183–193. [Google Scholar]
- 57.Jidoi, K., Nara, T. & Dodo, Y. (2001) Anthropol. Sci. 108, 345–370. [Google Scholar]
- 58.Frayer, D. W. (1993) Préhistoire Européene 2, 9–69. [Google Scholar]
- 59.Hublin, J. J. & Tillier, A. M. (1981) in Aspects of Human Evolution, ed. Stringer, C. B. (Taylor & Francis, London), pp. 167–185.
- 60.Tobias, P. V. (1967) in The Haua Fteah (Cyrenaica) and the Stone Age of the South-East Mediterranean, ed. McBurney, C. B. M. (Cambridge Univ. Press, Cambridge, U.K.), pp. 338–352.
- 61.Pinhasi, R. (1998) Dent. Anthropol. 12, 1–10. [Google Scholar]
- 62.Frayer, D. W. (1978) Univ. Kans. Publ. Anthropol. 10, 1–201. [Google Scholar]
- 63.Vallois, H. V. (1958) Gallia Suppl. 9, 118–137. [Google Scholar]