Abstract
Advanced age is associated with reduced brain levels of long-chain polyunsaturated fatty acids, arachidonic acid (AA) and docosahexaenoic acid (DHA). Memory impairment is also a common phenomenon in this age. Two-year-old, essential fatty acid-sufficient rats were fed with fish oil (11% DHA) for 1 month, and fatty acid as well as molecular composition of the major phospholipids, phosphatidylcholine and phosphatidylethanolamine (PE), was compared with that of 2-month-old rats on the same diet. DHA but not AA was significantly reduced in brains of old rats but was restored to the level of young rats when they received rat chow fortified with fish oil. This effect was pronounced with diacyl 18:0/22:6 PE species, whereas levels of 18:1/22:6 and 16:0/22:6 remained unchanged in all of the three PE subclasses. Fish oil reduced the AA in the old rat brains, diacyl and alkenylacyl 18:0/20:4 PE being most affected. Phosphatidylcholines gave less pronounced response. Six genes were up-regulated, whereas no significant changes were observed in brains of old rats receiving fish oil for 1 month. None of them except synuclein in young rat brains could be related to mental functions. Old rats on the fish-oil diet did not perform better in Morris water maze test than the control ones. A 10% increase in levels of diacyl 18:0/22:6 PE in young rat brains resulted in a significant improvement of learning capacity. The results are interpreted in terms of the roles of different phospholipid molecular species in cognitive functions coupled with differential responsiveness of the genetic machinery of neurons to n-3 polyunsaturated fatty acids.
Brain is one of the organs rich in phospholipids, which provide the building blocks for different membrane structures. These phospholipids are rather rich in long-chain polyunsaturated fatty acids, particularly in docosahexaenoic acid (DHA) and arachidonic acid (AA). DHA content of brain phospholipids seems to be precisely controlled. It is determined during pregnancy and early postnatal life (1, 2). There is a consensus that after this time it is almost impossible to alter brain fatty acid composition of adult, essential fatty acid-sufficient rats. On the other hand, any imbalance in polar head group or fatty-acid composition of structural lipids might have consequences on mental performance. Loss in DHA in brains of persons with Alzheimer's disease is accompanied with loss of memory and learning (3). In aged persons, impairment of memory also often takes place, and this is accompanied with loss of DHA in their brains (4–6). It has been shown that chronic administration of DHA to essential fatty acid-deficient young rats restores DHA levels in brain and improves memory (7). Similarly, DHA administration to ischemic rats had beneficial effect on spatial cognitive deficit (8). Moriguchi et al. (9) showed also that full recovery of brain DHA in essential fatty acid-deficient rats was obtained after 8 weeks on a DHA-containing diet. Hashimoto et al. (10) demonstrated that DHA provided protection from impairment of learning ability in rats with Alzheimer's disease, and McGahon et al. (11) showed that dietary DHA improved long-term potentiation in dentate gyrus of rat brain. Because the total fatty acid compositions of phospholipids were determined in most of these studies it seemed interesting to carry out detailed investigations on the impact of dietary fatty acids, rich in DHA, on the molecular composition of brain phospholipids and relate these data with mental performance. However, because it can be hypothesized that these functions are more complex than a simple function of brain lipid composition, effect of DHA on gene expression in aged rats was also studied.
Materials and Methods
Animals and Diet. Two- and 24-month-old rats were used in this study. Rats were fed either commercial rat chow (16:0 = 13.6%, wt/wt, of total fatty acids, 18:0 = 3.1%, 18:1, = 17.0%, 18:2 = 51.0%, 20:4 = 0.1%, and 22:6 = 1.2%) or rat chow supplemented with fish oil (16:0 = 13.4%, 18:0 = 7.1%, 18:1 = 23.0%, 18:2 = 21.2%, 20:4 = 0.8%, 20:5 = 2.8%, and 22:6 = 11.2%). Brains were snap-frozen and stored at –70°C until processing.
Analysis of Lipids. Lipids were extracted according to Folch et al. (12). Fatty acid composition of choline and ethanolamine phosphoglycerides separated by thin-layer chromatography according to Fine and Sprecher (13) was determined by gas chromatography using an FFAP column (30 m, 0.25 mm i.d., Supelco). Molecular species composition of choline and ethanolamine phosphoglycerides was determined as described in detail by Takamura and Kito (14). Diaryl glycerides, obtained by phospholipase C digestion using Bacillus cereus lipase (Sigma), were anthroxylated, and the derivatives were separated into phosphatidylethanolamine (PE) subclasses by thin-layer chromatography. Acetonitrile/isopropyl alcohol (75:25) as solvent was used to resolve the individual molecular species by HPLC. Derivatized 12:0/12:0 diglyceride was used as secondary standard.
Learning Test. Spatial learning was studied after the fourth week of dietary regime by using the Morris water escape task (15). During each of five daily sessions the animals received four trials. The escape latency reaching the hidden platform was registered at each trial. The escape platform was located always in the same quadrant of the circular swimming pool. The trial was terminated as soon as the rat climbed on the escape platform or when 90 s had elapsed, whichever occurred first. The daily mean escape latencies and the latencies of the first trial at each daily session were applied to statistical analysis. The latency to reach the hidden platform at the first trial reflects the reference memory best; therefore, this measure is shown in the present work.
Gene Expression. Gene expression was studied by DNA microarray technique as described (16–19). Briefly, a linear sample amplification protocol was used to amplify total RNA isolated from rat brain regions by using a NucleoSpin RNA purification kit (Macherey & Nagel). For generating fluorescent-labeled probes, random-priming-directed reverse transcription was used. After purification of labeled probes (from both treated and control animals), they were applied onto an in-house spotted 3,200 rat DNA microarray, hybridized for 16 h at 42°C, washed, and scanned by using a ScanArray Lite scanning confocal fluorescent scanner (GSI Lumonics, Billerica, MA) with 10-nm resolution.
A measure, i.e., “expression ratio,” denoting the median of the set of background-corrected single-pixel intensity ratios of Cy3 and Cy5 channels within the spot was determined by using the scanalyze2 software (www.microarrays.org/software.html). The calculated average expression ratio for all features on the array was normalized to 1.0. For background corrections those data were considered as negatives for which the average intensity of the spot was smaller than two times the average background of the same area. Significant spots have >0.55 CHGTB2 values (denotes for the fraction of pixels in the spot >1.5 times the background) in both channels. Replica spots (on the same array) and replica experiments (two different arrays with “color-flip” labeled probes) resulted in four data points for every gene sample. Those spots were excluded from further analysis for which ratios of the replica spots had >2-fold differences. The same restriction was applied for the average ratios of the replica experiments.
In the case of seven genes, gene-expression changes observed from microarray experiments were confirmed by quantitative real-time PCR experiments using a RotorGene 2000 instrument (Corbett Research, Sydney) with gene-specific primers and SyberGreen protocol.
Statistics were calculated by using the one-way ANOVA method.
Results
Fatty Acids. Tables 1 and 2 show the fatty acid composition of choline and ethanolamine phosphoglycerides in brains of young control and 2-year-old rats, as well as of rats kept on a fish-oil diet for 1 month. AA and DHA levels were low in choline phosphoglycerides irrespective of the age and diet. However, there was a small increase in DHA content in fish oil-fed young rat brains.
Table 1. Fatty acid composition of choline phosphoglycerides in relation to diet and age in rat brain.
| Composition, % of total fatty acids
|
||||
|---|---|---|---|---|
| Fatty acid | YR | YR + FO | OR | OR + FO |
| 16:0 | 36.60 ± 1.41 | 38.23 ± 2.85 | 37.33 ± 4.86 | 38.81 ± 2.54 |
| 18:0 | 19.81 ± 2.74 | 16.60 ± 0.55 | 14.41 ± 2.05 | 14.57 ± 1.63 |
| 18:1n-9 | 23.63 ± 1.63 | 23.85 ± 2.12 | 27.04 ± 2.54 | 25.06 ± 3.66 |
| 18:2n-6 | 0.96 ± 0.27 | 1.31 ± 0.67 | 1.38 ± 0.44 | 1.50 ± 0.36 |
| 20:1n-9 | 1.98 ± 0.23 | 1.77 ± 0.57 | 1.24 ± 0.36 | 1.21 ± 0.16 |
| 20:4n-6 | 5.20 ± 0.96 | 5.20 ± 0.96 | 5.65 ± 0.81 | 4.78 ± 0.50 |
| 22:4n-6 | ND | ND | 0.73 ± 0.86 | 0.60 ± 0.70 |
| 22:6n-3 | 4.18 ± 1.06 | 5.39 ± 1.32 | 3.60 ± 0.68 | 3.72 ± 0.32 |
YR, young rats; OR, old rats; FO, fish oil; ND, not detected.
Table 2. Fatty acid composition of ethanolamine phosphoglycerides in relation to diet and age in rat brain.
| Composition, % of total fatty acids
|
||||
|---|---|---|---|---|
| Fatty acid | YR | YR + FO | OR | OR + FO |
| DMA 16:0 | 4.14 ± 1.2 | 3.46 ± 0.33 | 5.33 ± 0.38 | 4.65 ± 0.55 |
| 16:0 | 6.68 ± 2.19 | 5.71 ± 1.30 | 5.75 ± 1.56 | 5.52 ± 0.13 |
| 16:1n-7 | 0.68 ± 0.24 | 0.63 ± 0.15 | 0.73 ± 0.29 | 0.63 ± 0.15 |
| DMA 18:0 | 8.00 ± 0.24 | 7.83 ± 1.06 | 9.15 ± 0.35 | 9.29 ± 1.14 |
| DMA18:1n-9 | 2.91 ± 1.35 | 2.74 ± 0.34 | 3.53 ± 0.32 | 3.30 ± 0.87 |
| DMA18:1n-7 | 1.91 ± 1.91 | 2.08 ± 0.56 | 5.44 ± 0.53 | 5.59 ± 1.28 |
| 18:0 | 16.35 ± 2.74 | 15.32 ± 2.00 | 13.81 ± 0.74 | 13.83 ± 0.81 |
| 18:1n-9 | 12.89 ± 1.69 | 13.90 ± 1.63 | 13.22 ± 0.76 | 13.72 ± 0.56 |
| 18:1n-7 | 1.96 ± 0.41 | 2.31 ± 0.22 | 5.44 ± 0.53 | 5.59 ± 1.28 |
| 18:2n-6 | 0.77 ± 0.15 | 0.91 ± 0.63 | 0.83 ± 0.22 | 0.61 ± 0.20 |
| 20:1n-9 | 4.10 ± 0.09 | 3.45 ± 0.73 | 3.39 ± 0.33 | 3.69 ± 0.62 |
| 20:4n-6 | 9.37 ± 0.93 | 8.70 ± 0.71 | 10.92 ± 1.05 | 9.32 ± 0.57 |
| 22:4n-6 | 5.19 ± 0.85 | 5.12 ± 0.22 | 5.60 ± 0.38 | 5.05 ± 0.35 |
| 22:6n-3 | 17.22 ± 1.63a,b | 19.51 ± 0.67a | 15.97 ± 0.56b | 17.21 ± 0.54 |
YR, young rat; OR, old rat; FO, fish oil; DMA, dimethyl acetal; note 20:4n-6 is AA and 22:6n-3 is DHA. Values with identical superscripts are significantly different (P < 0.05).
Table 2 shows that the fish-oil diet resulted in an increase of DHA from 17.2% to 19.5% (P < 0.05) in ethanolamine phosphoglycerides in brains of young rats. AA was reduced from 9.3% to 8.7%, but the difference did not reach the significance level. There is a small but significant (P < 0.05) reduction in DHA level in ethanolamine phosphoglycerides of old rats compared with young ones (16.0% vs. 17.2%) (Table 2). Fish-oil feeding completely restored the DHA level (P < 0.01) and rendered it indistinguishable from that observed with young animals (17.21% vs. 17.22%). Interestingly, we found higher AA levels in the ethanolamine phosphoglyceride fraction of old rat brains (9.3% vs. 10.9%), but this was reduced from 10.92% to 9.32% (P < 0.01), and this value is identical with that obtained for young animals (Table 2). Increased levels of AA in ethanolamine phosphoglycerides in brains of old rats is a result of elevated levels in the 18:0/20:4 species (Table 3).
Table 3. Molecular species composition of diacyl phosphatidylcholines in relation to age and diet.
| Composition, % of total fatty acids
|
||||
|---|---|---|---|---|
| Fatty acids | YR | YR + FO | OR | OR + FO |
| 18:1/22:6 | 0.48 | 0.54 | 0.50 | 0.49 |
| 16:0/22:6 | 2.53 | 2.81 | 2.84 | 2.48 |
| 18:0/22:6 | 3.30 | 4.72 | 2.80 | 3.33 |
| 18:1/20:4 | 0.54 | 0.93 | 1.08 | 1.09 |
| 16:0/20:4 | 2.54 | 2.98 | 4.30 | 3.78 |
| 18:0/20:4 | 4.72 | 4.61 | 4.40 | 4.69 |
YR, young rats; OR, old rats; FO, fish oil.
Molecular Species. Only diacyl species of choline phosphoglycerides, consisting of 93–95% of the total, are shown in Table 3. Species 18:0/22:6 responded to the fish-oil diet with a small increase from 3.3% to 4.7% in brains of young rats. As expected from Table 1, levels of 18:0/22:6 were lower in brains of old rats but fish-oil feeding did not result in a significant accumulation of this species. There is a small increase in 18:0/20:4 in old rat brains receiving fish oil, but the difference did not reach the P < 0.05 significance level.
Because ethanolamine phosphoglycerides are the mixtures of three different subclasses, molecular species composition of diacyl, alkylacyl, and alkenylacyl subclasses was separately determined. The diacyl subclass makes up 42–45%, whereas alkylacyl and alkenylacyl subclasses make up 4–10% and 45–50%, respectively, of the total. In Table 4 molecular species composition of DHA-containing ethanolamine phosphoglyceride subclasses is given in relation to age and diet. The data on distribution of diacyl 18:0/22:6 species agree well with the fatty acid composition data; i.e., there is a significant (P < 0.05) reduction in its level in old rat brains. This comparison also clearly shows that dietary treatment brought about an increase in levels of diacyl 18:0/22:6 species from 28.9% to 32.9% in young rats (P < 0.05) and from 25.1% to 29.6% in old rats (P < 0.05). Diacyl 18:1/22:6 and 16:0/22:6 did not respond at all. Alkylacyl 18:1a/22:6, 16:0a/22:6, and 18:0a/22:6 (“a” indicates alcohol) responded differently in the two age groups. Fish oil brought about a decrease in young rat brains whereas it brought about an increase in its level in old rat brains. Only alkenylacyl 18:0a/22:6 in young rats responded with an increase from 15.2% to 17.1% (P < 0.05).
Table 4. DHA- and AA-containing phosphatidylethanolamine molecular species in rat brain in relation to age and diet.
| Composition, % of total molecular species
|
||||
|---|---|---|---|---|
| Species | YR | YR + FO | OR | OR + FO |
| Diacyl species (n = 6) | ||||
| 18:1/22:6 | 1.94 ± 0.85 | 1.88 ± 0.35 | 1.68 ± 0.30 | 1.84 ± 1.90 |
| 16:0/22:6 | 6.45 ± 0.95 | 6.69 ± 0.61 | 6.13 ± 1.05 | 6.92 ± 1.08 |
| 18:0/22:6 | 28.95 ± 1.90a | 32.98 ± 1.25a | 25.13 ± 1.38b | 29.62 ± 1.49b |
| 18:1/20:4 | 3.14 ± 0.80 | 2.19 ± 0.36 | 3.98 ± 1.46 | 2.74 ± 0.33 |
| 16:0/20:4 | 2.58 ± 0.82 | 1.78 ± 0.19 | 2.96 ± 1.73 | 2.29 ± 0.34 |
| 18:0/20:4 | 24.17 ± 2.48c | 18.99 ± 1.36c | 22.15 ± 1.91 | 20.04 ± 1.10 |
| Alkylacyl species (n = 6) | ||||
| 18:1a/22:6 | 3.29 ± 0.42 | 0.66 ± 0.26 | 3.69 ± 1.43 | 6.97 ± 1.28 |
| 16:0a/22:6 | 8.14 ± 2.70 | 1.42 ± 0.56 | 3.84 ± 2.98 | 9.37 ± 3.00 |
| 18:0a/22:6 | 12.15 ± 0.82 | 15.50 ± 0.95 | 9.34 ± 2.20d | 13.59 ± 1.53d |
| 18:1a/20:4 | 4.84 ± 0.84 | 4.40 ± 0.72 | 5.69 ± 2.44 | 4.82 ± 1.98 |
| 16:0a/20:4 | 5.02 ± 0.10 | 4.89 ± 0.13 | 5.90 ± 1.93 | 4.52 ± 1.38 |
| 18:0a/20:4 | 6.33 ± 1.66 | 9.77 ± 2.92 | 7.84 ± 2.81 | 5.60 ± 1.02 |
| Alkenylacyl species (n = 6) | ||||
| 18:1a/22:6 | 2.95 ± 0.70 | 4.05 ± 0.74 | 4.05 ± 0.62 | 4.42 ± 0.53 |
| 16:0a/22:6 | 6.28 ± 1.56 | 7.55 ± 1.27 | 7.95 ± 1.41 | 7.29 ± 1.47 |
| 18:0a/22:6 | 15.59 ± 0.90e | 17.83 ± 0.82e | 13.59 ± 1.09 | 14.39 ± 2.10 |
| 18:1a/20:4 | 4.63 ± 0.88 | 4.62 ± 0.91 | 6.95 ± 1.40 | 4.81 ± 0.68 |
| 16:0a/20:4 | 3.50 ± 1.04 | 3.61 ± 0.76 | 4.42 ± 0.75 | 2.90 ± 0.90 |
| 18:0a/20:4 | 8.42 ± 0.84 | 6.95 ± 0.62 | 7.92 ± 0.60 | 6.57 ± 0.31 |
, Alcohol; YR, young rats; OR, old rats; FO, fish oil. Values with identical superscripts are significantly different (P < 0.05).
Table 4 compiles the data obtained on AA-containing PE subclasses as well. The level of AA-containing diacyl 18:0/20:4 species was identical in the brains (24.1% vs. 22.1%). Fish-oil feeding brought about a significant (P < 0.05) decrease in the level of this species in young rats, and the same tendency was seen in old rats. Interestingly, alkylacyl 18:0a/20:4 accumulated AA in fish-oil-fed young rats (from 6.3% to 9.7%), whereas the opposite trend was observed with old rats. Molecular species composition of alkenylacyl subclasses was almost identical in young and old rats and remained unaffected by the diet.
Table 5 summarizes the overall distribution of different ethanolamine phosphoglyceride subclasses in relation to diet and age. This approach clearly shows the differences between the young and old rats and the differential effect of the fish-oil diet on brain-lipid composition. The level of total DHA-containing diacyl PE subclasses was lower in brains of old rats (33% vs. 37.3%). Fish-oil feeding brought about an increase of their levels in both young and old rat brains in a manner in which their levels in old rat brains become identical with that in young rat brains receiving the control diet (38.3% vs. 37.3%). This approach also shows that a large amount of 22:6 was accumulated in alkylacyl subclass of fish oil-fed old rat brains (16.8% vs. 29.5%) in contrast to young rats, where the opposite effect was observed. The alkenylacyl subclass remained silent. The diacyl AA-containing subclasses responded to the fish-oil diet with a decrease in their levels. The alkenylacyl subclasses remained virtually unaltered, but the alkylacyl subclasses gave an opposite response: there was an increase in young rat brains and a decrease in old rat brains.
Table 5. Overall distribution of phosphatidylethanolamine subclasses in relation to age and diet in rat brain.
| Composition, % of total molecular species
|
||||
|---|---|---|---|---|
| Subclass | YR | YR + FO | OR | OR + FO |
| X/22:6 | ||||
| Diacyl | 37.34 | 41.73 | 32.93 | 38.38 |
| Alkylacyl | 23.78 | 11.01 | 16.82 | 29.52 |
| Alkenylacyl | 25.89 | 27.53 | 24.83 | 26.08 |
| X/20:4 | ||||
| Diacyl | 29.34 | 22.18 | 29.23 | 25.38 |
| Alkylacyl | 13.58 | 19.00 | 19.37 | 14.94 |
| Alkenylacyl | 17.29 | 17.11 | 18.83 | 14.34 |
X, 18:1 + 16:0 + 18:0; YR, young rats; OR, old rats; FO, fish oil.
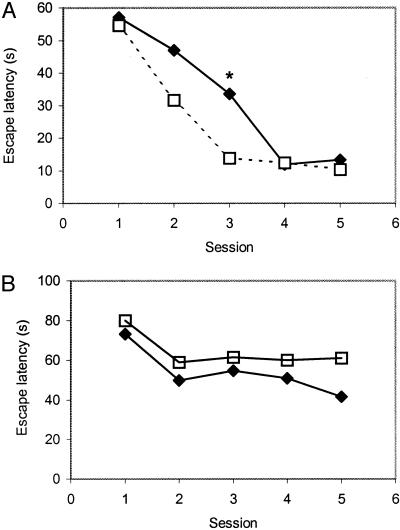
Spatial Learning. Fig. 1A shows that young rats on fish oil tended to perform better in Morris water maze test than the control ones the difference reached the P < 0.05 significance level at the third session (t = 2.24, df = 17, P < 0.05). In contrast, the old rats fed the fish-oil diet did not show improvement in their spatial memory (Fig. 1B), indicating that elevated levels of DHA might not have been sufficient to evoke the known beneficial effect of n-3 polyunsaturated fatty acids on learning. Performance was also not different from that of the old rats when the main latencies per session were computed (data not shown). Furthermore, when we evaluated the daily improvement during the test, we found that young rats showed a highly significant learning effect throughout the course of five daily sessions (F4,68 = 49.03, P < 0.0001), whereas aged rats just reached significance in improvement of their daily memory performance (F4,56 = 3.88, P < 0.05, ANOVA with repeated measures).
Fig. 1.
Morris water maze performance of 3-month-old rats (A) and 2-year-old rats (B) after 1 month of a DHA-enriched diet. *, P < 0.05. (A) ♦, Young control; - - □ - -, young fish oil. (B) ♦, Old control; —□—, old fish oil.
Gene Expression. Because earlier we showed that a fish-oil diet brought about an overexpression of a number of genes in young rats, gene expression was also studied in this experiment. Actually, 55 genes were found overexpressed and 47 genes repressed in brains of rats receiving fish oil from conception until adulthood (16). In contrast, only six genes were up-regulated and no gene appeared to be repressed in brains of young rats receiving fish oil for only 1 month (Table 6). Among the up-regulated genes was the gene encoding the α-synuclein and transthyretin. In brains of old rats receiving identical diets there was no significant change in the gene expression patterns (Table 7).
Table 6. Changes in gene expression in brain of 2-month-old rats receiving a DHA-enriched diet for 1 month.
| Average change, -fold | Gene product | GenBank accession no. |
|---|---|---|
| 0.534 | CDC10 gene exon 13 (Mus musculus) | AW539811 |
| 0.547 | Heat shock protein 86 kDa (M. musculus) | AW536206 |
| 0.565 | Chromosome 14 clone CTD-2547F10 (Homo sapiens) | AW544599 |
| 0.569 | DEK oncogene (DNA binding) (H. sapiens) | AW539649 |
| 0.580 | DHA chromosome 4, BAC clone (Arabidopsis thaliana) | AW544613 |
| 0.582 | H3 histone, family 3B (H3f3b) (M. musculus) | AW539780 |
| 0.589 | Chromosome 7 clone UWGC:G1564a3 (H. sapiens) | AW539673 |
| 0.593 | ESTs, similar to Golgi peripheral membrane protein | AA213185 |
| 0.594 | No hits found | AA410137 |
| 0.598 | Ribosomal protein S15 (Rps15) (M. musculus) | AW544444 |
| 0.602 | Chromosome 19, cosmid R33729 (H. sapiens) | AW407543 |
| 0.604 | Protein kinase C inhibitor (mPKCI) (M. musculus) | AW544588 |
| 0.606 | Ras-related GTP-binding protein (H. sapiens) | AW539762 |
| 0.612 | SRY-related protein SOX 8 (M. musculus) | AW544622 |
| 0.624 | cDNA FLJ10537 fis, clone NT2RP200 (H. sapiens) | AW536241 |
| 0.627 | Ribosomal protein L23 | AW540935 |
| 0.630 | Pfkfb2 gene, exons 1-15 (H. sapiens) | AW539613 |
| 0.632 | Transmembrane 9 superfamily (H. sapiens) | AW540938 |
| 1.925 | Transthyretin | W17647 |
| 1.994 | Mesoderm development candiate 2 (M. musculus) | AA087768 |
| 2.557 | Similar to kinesin light chain KLCt | W15957 |
| 2.818 | cDNA clones IMAGE:331327 5′ (M. musculus) | W14581 |
| 3.785 | Interferon regulatory factor (H. sapiens) | W41937 |
| 8.638 | α-Synuclein mRNA | W41663 |
Table 7. Changes in gene expression in brain of 2-year-old rats receiving a DHA-enriched diet for 1 month.
| Average change, -fold | Gene product | GenBank accession no. |
|---|---|---|
| 0.449 | Mitochondrion genome | AW545415 |
| 0.531 | Zinc finger protein BERF-1 (M. musculus) | AA052449 |
| 0.535 | BAC clone RP11-554J4 (H. sapiens) | AA267057 |
| 0.551 | Fibroblast growth factor, intracellular | W18449 |
| 0.572 | T cell acute lymphocytic leukemia 1 | AA270232 |
| 0.582 | No hits | AA285972 |
| 0.588 | cDNA DKFZp434N2412 | AA409778 |
| 0.618 | No hits | AA259386 |
| 0.620 | Transforming growth factor β, induced, 68 kDa | AA268592 |
| 0.623 | mGpi1 gene, exon 10, tubby gene (M. musculus) | AA273704 |
| 1.515 | Similar to gb:s87759 protein phosphatase 2C | W18642 |
| 1.514 | Low-density lipoprotein receptor-related protein | AA253890 |
| 1.512 | rhoC | AA221997 |
| 1.634 | No hits | AA276290 |
| 1.648 | ESTs, highly similar to mitochondrial elongation factor | AA245481 |
| 1.707 | Ubiquitin-conjugating enzyme E2 | AA261605 |
| 1.726 | Tnfa-induced adipose-related protein (M. musculus) | AA272372 |
| 1.864 | ESTs, weakly similar to C43E11.9 (Caenorhabditis elegans) | AA274849 |
| 2.238 | Hypothetical | AA270649 |
Discussion
DHA- and AA-containing phosphoglycerides have been proposed to play a special role in biomembranes including those of neurons. This fact is due to their favorable biophysical properties such as high motility and motional freedom (20–23). DHA-containing phospholipid molecular species might affect the blood–brain barrier (24), neurotransmission (4, 22, 25), or ionic channels (26, 27). However, all of our knowledge on biophysical properties of membranes is based on results obtained on choline phosphoglycerides. As shown, these phospholipids did not respond to dietary intervention and thus might not have been involved in improved mental activities. The phospholipids responding to dietary fatty acids were the ethanolamine phosphoglycerides, particularly diacyl 18:0/22:6. Some biophysical parameters of ethanolamine phosphoglycerides differ from those of choline phosphoglycerides. For instance, in model experiments, diacyl PEs, in contrast to sn-1 saturated/sn-2 unsaturated diacyl phosphatidylcholines, tended to increase membrane packing (28); thus, changes in the molecular architecture and biophysics of neural membranes might be the opposite of those expected based on choline phosphoglycerides. In addition, ethanolamine phosphoglycerides are cone-shaped and are prone to form so-called nonbilayer phases, in contrast to choline phosphoglycerides, which adapt a cylindrical shape and do not form nonbilayer phases. The importance of propensity to form nonbilayer phases of diacyl PEs in binding G proteins has already been demonstrated (29, 30) and has suggested a role of DHA-containing phospholipids in G protein-coupled signaling pathways. Furthermore, choline phosphoglycerides enrich the outer leaflet of membrane bilayer whereas ethanolamine phosphoglycerides enrich the inner leaflet.
Brain is known to retain DHA for a long period; to reduce its level in ethanolamine phosphoglycerides, the main pool of this fatty acid, at least two generations are needed. Aging is a physiological process whereby DHA level diminishes in brain phospholipids, first of all in ethanolamine phosphoglycerides (5, 6). Loss of DHA in aged individuals is connected with a decline of cognitive functions such as learning and memory. It is tempting to speculate that this decline in memory can be paralleled with reduced levels of diacyl 18:0/22:6 PE. The present results show that DHA level can be restored in essential fatty acid-sufficient old rats to a normal level within a relatively short period: in our experiments 1 month of fish-oil feeding was sufficient to increase DHA in all of the three subclasses of ethanolamine phosphoglycerides (Table 3). Young rats on a DHA-poor diet for two generations needed 8 weeks to partially restore brain DHA when kept on an n-3-adequate diet (9). McGahon et al. (11) also found that a 2-month feeding period was necessary to recover the total amount of DHA in rat brain synaptic vesicles. Evidently, in our case the difference in DHA level between the control and experimental animals was less than in the above two cases, and this might explain the complete recovery of DHA in ethanolamine phosphoglycerides within 1 month.
AA is the second polyunsaturated fatty acid that might be affected during aging. We demonstrated in this study a slight but insignificant increase in AA level in total fatty acids (Table 2), which might be due to an increase in levels of alkylacyl and alkenylacyl 18:1a/20:4 as well as alkenylacyl 16:0a/22:6 (Table 4) PE molecular species (Table 4). Fish-oil feeding resulted in a decrease in AA-containing species first of all in diacyl 18:0/20:4. McGahon et al. (11) showed that both DHA and AA supported long-term potentiation in hippocampus of rats kept on a fish-oil diet. In contrast to their results, in our experiments the aged rats kept on the fish-oil diet did not improve learning performance as did young rats. However, it should be considered that in our experiment the level of all of the PE subclasses containing AA in position sn-2 was slightly reduced by fish oil in aged rats (Table 3). DHA has been shown to replace AA in phosphoglycerides in various tissues, including brain.
The increase in level of DHA-containing ethanolamine phosphoglycerides in brains of fish oil-fed young rats was paralleled with a better performance in the Morris water maze test (Fig. 1A). In all of the experiments reported so far, animals raised for several generations on either essential fatty acid-deficient or sufficient diets were compared, and huge differences in brain DHA content were found (31–34). In those experiments, the diet restored DHA level and learning performance of essential fatty acid-deficient rats to the level of essential fatty acid-sufficient rats. In our experiment, level of DHA and DHA-containing PEs increased only by 10–15%. In a previous experiment in which the rats were kept on a fish-oil diet from conception until reaching adulthood, levels of diacyl 18:0/22:6 were 34.1% (16) compared with 33.0% in the present study and with 30% in the control rats (Table 4). Thus, this small increase in DHA levels appeared to be sufficient to produce a detectable effect on the reference memory of young rats. However, AA-containing PE molecular species do not seem to play a role in the observed improvement of the reference memory, because their levels significantly decreased at least in diacyl and alkenylacyl subclasses even more than in old rats (24.1% vs. 19% and 8.4% vs. 7.0% in young rats, and 22.1% vs. 20.0% and 7.9% vs. 6.5% in old rats, respectively; Table 4).
In addition, it is interesting to speculate that the genetic machinery of brain might also be involved in this kind of response. In a previous study (16), we found that several genes possibly related to cognitive functions (such as synucleins, the ATP-generating machinery, synaptic plasticity, signal transduction, ion channel formation, etc.) were overexpressed in brains of young rats receiving fish oil from conception until adulthood. In brains of young rats on fish oil, just for 1 month, only synuclein was overexpressed in addition to transthyretin, which was down-regulated in the previous experiment (16). Synucleins were shown to accumulate in brains of songbirds during the period of song learning (35), whereas transthyretin binds thyroid hormone and thus prevents its insertion into neural membranes (36) and prevents amyloid formation (37). In old rats none of these genes responded. These results suggest that brain responds to n-3 fatty acids in an age- and feeding-time-dependent manner. However, it is unclear at the present stage of experiments whether DHA interacts directly with the genetic machinery of neurons or its effects on gene expression pattern and molecular architecture of membranes are unrelated events. From the fact that fish oil-fed old rats failed to perform better in a learning test (Fig. 1B) one may conclude that elevated level of DHA or DHA-containing PEs alone cannot be made responsible for their observed beneficial effect on mental activities. Based on these observations, we put forth the hypothesis that, besides the elevated level of these molecular species and altered molecular architecture of neural membranes, a number of genes, stimulated in young rats by these fatty acids in short- or long-term feeding experiments, but remaining silent in old rats in short-term feeding experiment, concertedly take part in supporting cognitive processes.
Acknowledgments
Fish oil was a generous gift from B. L. T. Berg (Lipidtech, Aale, Norway). This research was supported by a grant from the Hungarian National Research and Development Initiative (1/040/2001), by the Hungarian Research Council (OTKA T034895, OTKA 38388, F-042850, T038 388, TS 044836), and by EC QLRT-2001001172. K.K. and L.G.P. were supported by a János Bolyai scholarship.
Abbreviations: AA, arachidonic acid; DHA, docosahexaenoic acid; PE, phosphatidylethanolamine.
References
- 1.Carrie, I., Clement, M., De Javel, D., Frances, H. & Bourre, J. M. (2000) J. Lipid Res. 41, 465–472. [PubMed] [Google Scholar]
- 2.Schiefermeier, M. & Yavin, E. (2002) J. Lipid Res. 43, 124–131. [PubMed] [Google Scholar]
- 3.Soderberg, M., Edlund, C., Kristensson, K. & Dallner, G. (1991) Lipids 26, 421–425. [DOI] [PubMed] [Google Scholar]
- 4.Delion, S., Chalon, S., Guilloteau, D., Besnard, J.-C. & Durand, G. (1996) J. Neurochem. 66, 1582–1591. [DOI] [PubMed] [Google Scholar]
- 5.Delion, S., Chalon, S., Guillotean, D., Lejeune, B., Besnard, J. C. & Durand, G. (1997) J. Lipid Res. 38, 680–689. [PubMed] [Google Scholar]
- 6.Favreliere, S., Stadelaman-Ingrand, S., Huguet, F., De Javel, D., Piriou, A., Tallineau, C. & Durand, G. (2000) Neurobiol. Aging 21, 653–660. [DOI] [PubMed] [Google Scholar]
- 7.Gamoh, S., Hashimoto, M., Sugioka, K., Hossain, M. S., Hata, N., Mishava, Y. & Masumura, S. (1999) Neuroscience 93, 237–241. [DOI] [PubMed] [Google Scholar]
- 8.Okada, M., Amamoto, T., Tomonaga, M., Kawachi, A., Yazawa, K., Mine, K. & Fujiwara, M. (1996) Neuroscience 71, 17–25. [DOI] [PubMed] [Google Scholar]
- 9.Moriguchi, T., Loewke, J., Garrison, M., Catalan, J. N. & Salem, N., Jr. (2001) J. Lipid Res. 42, 419–427. [PubMed] [Google Scholar]
- 10.Hashimoto, M., Hossain, S., Shimada, T., Sugioka, K., Yamasaki, H., Fuji, Y., Ishibashi, Y., Oka, J.-I. & Shido, O. (2002) J. Neurochem. 81, 1084–1091. [DOI] [PubMed] [Google Scholar]
- 11.McGahon, B. M., Martin, D. S. D., Horrobin, D. F. & Lynch, M. A. (1999) Neuroscience 94, 305–314. [DOI] [PubMed] [Google Scholar]
- 12.Folch, J., Lees, M. & Sloane-Stanley, G. H. (1957) J. Biol. Chem. 226, 497–509. [PubMed] [Google Scholar]
- 13.Fine, J. B. & Sprecher, H. (1982) J. Lipid Res. 13, 660–663. [PubMed] [Google Scholar]
- 14.Takamura, H. & Kito, M. (1991) J. Biochem. 109, 436–439. [DOI] [PubMed] [Google Scholar]
- 15.Eijkenboom, M. & van der Staay, F. J. (1999) Neuroscience 91, 1299–1313. [DOI] [PubMed] [Google Scholar]
- 16.Kitajka, K., Puskás, L. G., Zvara, Á., Hackler, L., Jr., Barceló-Coblijn, G., Yeo, Y. K. & Farkas, T. (2002) Proc. Natl. Acad. Sci. USA 99, 2619–2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Puskás, L. G., Zvara, A., Hackler, L., Jr., & van Hummelen, P. (2002) BioTechniques 32, 1330–1340. [DOI] [PubMed] [Google Scholar]
- 18.Puskás, L. G., Zvara, A., Hackler, L., Jr., Micsik, T. & van Hummelen, P. (2002) BioTechniques 33, 898–904. [DOI] [PubMed] [Google Scholar]
- 19.Eisen, M. B., Spellman, P. T., Brown, P. O. & Botstein, D. (1998) Proc. Natl. Acad. Sci. USA 95, 14863–14868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Salem, N., Jr., & Niebylski, C. D. (1995) Mol. Membr. Biol. 12, 131–134. [DOI] [PubMed] [Google Scholar]
- 21.Litman, B. J. & Mitchel, D. C. (1996) Lipids 37, S193–S197. [DOI] [PubMed] [Google Scholar]
- 22.Kim, H.-Y. & Edsall, L. (1999) Lipids 34, S249–S250. [DOI] [PubMed] [Google Scholar]
- 23.Salem, N., Jr., Litman, B., Kim, H.-Y. & Gawrisch, K. (2001) Lipids 36, 945–950. [DOI] [PubMed] [Google Scholar]
- 24.Hussain, S. T. & Roots, B. I. (1994) Biochem. Soc. Trans. 2, 338S. [DOI] [PubMed] [Google Scholar]
- 25.Minami, M., Kimura, S., Endo, T., Hamaue, N., Hiraguji, M., Togashi, H., Matsumoto, N., Yoshioka, M., Saito, H., Watanabe, S., et al. (1997) Pharmacol. Biochem. Behav. 58, 1123–1129. [DOI] [PubMed] [Google Scholar]
- 26.Vreugdenhil, M., Bruehl, C., Voskuyl, R. A., Kang, J. X., Leaf, A. & Wadman, W. J. (1996) Proc. Natl. Acad. Sci. USA 93, 12559–12563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nishikawa, M., Kimura, S. & Akaike, N. (1994) J. Physiol. (London) 475, 83–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fodor, E., Johnes, R. H., Buda, C., Kitajka, K., Dey, I. & Farkas, T. (1996) Lipids 30, 1119–1126. [DOI] [PubMed] [Google Scholar]
- 29.Escriba, P. V., Ozaita, A., Ribas, C., Miralles, A., Fodor, E., Farkas, T. & Garcia-Sevilla, J. A. (1997) Proc. Natl. Acad. Sci. USA 94, 11375–11380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Litman, B. J., Niu, S. L., Polozova, A. & Mitchell, D. C. (2001) J. Mol. Neurosci. 16, 237–242. [DOI] [PubMed] [Google Scholar]
- 31.Yamamoto, N., Saitoh, M., Moriuchi, A., Nomura, M. & Okuyama, H. (1987) J. Lipid Res. 28, 144–151. [PubMed] [Google Scholar]
- 32.Frances, H., Monier, C., Clement, M., Lecorsier, A., Debray, M. & Bourre, J. M. (1996) Life Sci. 21, 1805–1816. [DOI] [PubMed] [Google Scholar]
- 33.Carriè, I., Clèment, M., De Javel, D., Francès, H. & Bourre, J. M. (2000) J. Lipid Res. 41, 473–480. [PubMed] [Google Scholar]
- 34.Weisinger, H. S., Vingrys, A. J. & Sinclair, A. J. (1995) Lipids 30, 471–473. [DOI] [PubMed] [Google Scholar]
- 35.George, J. M., Jim, H., Woods, W. S. & Clayton, D. F. (1995) Neuron 15, 361–372. [DOI] [PubMed] [Google Scholar]
- 36.Schreiber, J. M., Jim, H., Woods, W. S. & Clayton, D. F. (1995) Neuron 15, 361–372. [DOI] [PubMed] [Google Scholar]
- 37.Schwarzman, A. L., Gregori, L., Vitek, M. P., Lyubski, S., Strittmatter, W. J., Enghilde, J. J., Bhasin, R., Silverman, J., Weisgraber, K. H., Coyle, P. K., et al. (1994) Proc. Natl. Acad. Sci. USA 91, 8368–8372. [DOI] [PMC free article] [PubMed] [Google Scholar]