Abstract
Fragmentation of various extracellular matrix and blood proteins generates antiangiogenic substances that are physiological regulators of angiogenesis. Some of these compounds are in clinical trials as inhibitors of tumor angiogenesis. Anastellin, an antiangiogenic protein fragment derived from fibronectin, was unable to inhibit matrigel plug angiogenesis in mice that lack plasma fibronectin. Anastellin was fully active in mice that are null for vitronectin, which, like fibronectin, is a major adhesion protein in the blood. An antiangiogenic form of antithrombin showed the opposite pattern. The activity of endostatin was impaired in both fibronectin- and vitronectin-deficient mice. These results suggest a shared mechanism of action for antiangiogenic factors derived from extracellular matrix and plasma proteins: these factors form complexes with adhesion proteins in plasma to create an active antiangiogenic substance.
Agrowing class of antiangiogenic substances is derived from extracellular matrix and blood proteins by proteolysis or other modifications. These substances include fragments from thrombospondin (1), plasminogen (angiostatin; ref. 2), collagen type XVIII (endostatin; ref. 3), collagen type IV (tumstatin; ref. 4), a modified form of antithrombin (5), and the fibronectin fragment anastellin (6, 7). The molecular mechanisms whereby these substances exert their antiangiogenic activities are poorly understood. Anastellin binds to and polymerizes fibronectin and fibrinogen (8, 9). The antiangiogenic form of antithrombin is similar to the modified antithrombin that binds vitronectin (10, 11). Vitronectin, like fibronectin, is an arginine-glycine-aspartic acid (RGD)-containing adhesion protein present in plasma (12). Other angiogenesis inhibitors also interact with one or more adhesion proteins: angiostatin and its parent protein, plasminogen, bind vitronectin (13), whereas endostatin binds fibulins and nidogen-2 (14).
The interactions of the various angiogenesis inhibitors with adhesion proteins led us to hypothesize that adhesion protein binding could underlie antiangiogenic activity, and that activities of the various inhibitors could converge on this ability to form such adhesion protein complexes (7). To test this hypothesis, we examined the two antiangiogenic proteins with the most extensively documented adhesion protein interactions, anastellin and antithrombin. We used angiogenesis induced by implanting matrigel (basement membrane) plugs (15, 16) into mutant mice that conditionally lack plasma fibronectin (17) or have their vitronectin gene knocked out (18).
We show here that anastellin does not suppress angiogenesis in matrigel plugs implanted into mice that lack plasma fibronectin. In contrast, the antiangiogenic form of antithrombin is inactive in mice that lack vitronectin. Endostatin is essentially inactive in mice that lack either plasma fibronectin or vitronectin. These results strongly support the hypothesis that the activity of extracellular matrix- and blood protein-derived angiogenesis inhibitors depends on interactions with adhesion proteins.
Materials and Methods
Proteins. Anastellin (a fragment from the first type III repeat in human fibronectin) and III11-C (control fragment from the 11th type III repeat of fibronectin) were prepared as recombinant his-tagged proteins in bacteria and purified as described (6, 17). Human plasma fibronectin and human vitronectin were from Chemicon (Temecula, CA), and human fibrinogen was from Sigma. The protein solutions were sterilized by filtering through a 0.2-μm membrane before experimentation. Recombinant human endostatin and human plasma antithrombin III were from Calbiochem. Antithrombin III was treated with guanidine·HCl to convert the native protein to its antiangiogenic form as described (5). This form of the protein is henceforth referred to as antithrombin.
Mice. Two-month-old immunodeficient female BALB/c/nu/nu mice (Harlan–Sprague–Dawley, San Diego) were used to establish the model for matrigel angiogenesis inhibition. Two plasma fibronectin Cre-loxP conditional knockout mouse lines [Fn(fl/fl); Alb-Cre+, and Fn(fl/fl); Mx-Cre+] (17) were used. In the Fn(fl/fl); Alb-Cre+ mice, the Cre expression is under the control of the albumin promoter and causes postnatal elimination of the fibronectin gene in the liver, which is the source of essentially all plasma fibronectin. The Fn(fl/fl); Mx-Cre+ line expresses Cre in an IFN-inducible manner. Deletion of the fibronectin gene in the mice was induced in 4- to 5-week-old mice as described (17). These mice have been shown to express <0.04% of the normal plasma fibronectin level (17). A vitronectin-null mouse line (18) was kindly provided by David Loskutoff (The Scripps Research Institute, La Jolla, CA). Wild-type C57BL/6J mice were used as controls for the vitronectin knockout mice. All knockout mice were genotyped. Immunoblotting performed at the time the mice were used in the experiments (4 months of age) showed no detectable fibronectin in the plasma of the mice with deleted fibronectin genes; their plasma vitronectin levels were normal. The vitronectin knockout mice were negative for plasma vitronectin, but had normal plasma fibronectin levels (results not shown).
Matrigel Angiogenesis Assay. Matrigel was from Becton Dickinson. Recombinant human basic fibroblast growth factor (bFGF) and recombinant mouse vascular endothelial growth factor (VEGF) were from R & D Systems. The rat anti-mouse CD31 antibody was from Pharmingen. Liquid matrigel containing 100 ng of bFGF or 50 ng of VEGF per ml was injected s.c. in the abdominal region of the mouse. Each mouse received one, or usually two, 0.5-ml matrigel plugs.
The mice were treated with daily i.p. injections of one of the angiogenesis inhibitors in 0.3 ml of PBS, or PBS as a control. A fragment corresponding to the homologous residues from the 11th type III domain of human fibronectin was used as a treatment control for anastellin (6). After 7–10 days, the mice were killed and the matrigel plugs were removed. The matrigel plugs were fixed in 4% paraformaldehyde, stored in 70% ethanol, and used to cut sections. Paraffin embedding, sectioning, and immunostaining of the plugs for CD31 and other blood vessel markers were carried out in The Burnham Institute's Histology Facility or at Pharmingen. Blood vessels were counted in an average of three sections from each matrigel plug. Alternatively, the plugs were homogenized and their hemoglobin content was determined by using the Drabkin reagent kit (Sigma). Student's t test was used in statistical analysis of the results.
Results
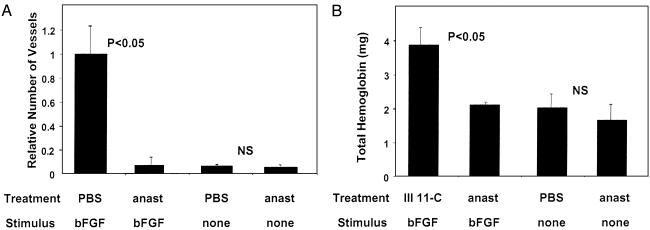
To validate the matrigel model, we implanted mice with s.c. matrigel plugs that were supplemented with either bFGF or vascular endothelial growth factor, and treated the mice with i.p. injections of anastellin. The number of blood vessels and total hemoglobin content in the plugs correlated with the amount of the angiogenic factor added to the gel. Anastellin treatment essentially eliminated the angiogenic effect of both substances, but had no effect on the basal level of blood vessel formation in plugs that received no growth factor. A control fragment analogous to anastellin, but derived from another fibronectin type III (11th type III) domain, was inactive. Based on the results exemplified in Fig. 1, we chose 50 ng of bFGF as the angiogenic stimulus for the testing of the adhesion protein dependence of three angiogenesis inhibitors: anastellin, antithrombin, and endostatin.
Fig. 1.
In vivo angiogenesis assay in matrigel plugs. Mice bearing matrigel plugs impregnated with bFGF were treated with daily i.p. injections of 1 mg of anastellin (anast), PBS, or a control fragment homologous to anastellin from the 11th type III domain of fibronectin for 10 days. Angiogenesis was evaluated by counting the number of blood vessels in the plugs (A) or by measuring the hemoglobin content in duplicate plugs (B). Combined results from three independent experiments, with three or four mice in each test group, are shown. The P values show the significance level of the differences observed between the indicated test groups. NS, not significant.
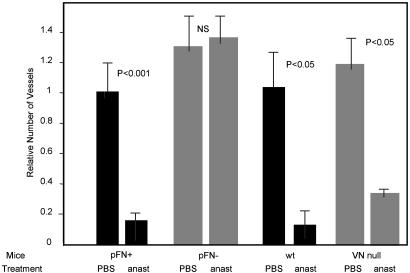
Anastellin had no antiangiogenic activity in the fibronectin-deficient mice, but was fully active in their normal littermates. The results obtained by determining blood vessel density in tissue sections (Fig. 2) were confirmed by measuring the amount of hemoglobin in the plugs (Table 1). In contrast, anastellin was fully active in vitronectin-null mice (Fig. 2). Thus, plasma fibronectin is necessary for the antiangiogenic activity of anastellin, but vitronectin is not.
Fig. 2.
Anastellin activity requires plasma fibronectin, but not vitronectin. Mice deficient in plasma fibronectin (pFN–), their littermate controls (pFN+), vitronectin-null mice (VN null), and same strain wild-type (wt) controls for the VN null mice were injected with matrigel plugs impregnated with bFGF. The mice were then treated with seven daily i.p. injections of 1 mg of anastellin or PBS. The number of blood vessels in the plugs is shown. The two fibronectin-deficient lines gave similar results; combined results from three experiments with 56 mice are shown. Twelve mice were used to study the role of vitronectin. The P values show the significance level of the differences observed between the indicated test groups. NS, not significant.
Table 1. Effect of angiogenesis inhibitors on the hemoglobin content of matrigel pellets in mice lacking plasma fibronectin or vitronectin.
| Mouse line
|
||||
|---|---|---|---|---|
| Treatment | pFN+ | pFN- | wt | VN null |
| PBS | 8.6 ± 1.2 | 7.2 ± 0.8 | 5.1 ± 0.2 | 6.3 ± 0.8 |
| Anastellin | 2.5 ± 0.3** | 7.6 ± 1.2 | 2.0 ± 0.2** | 2.4 ± 0.6** |
| PBS | 7.8 ± 0.7 | 7.2 ± 0.4 | 6.2 ± 0.4 | 6.5 ± 0.4 |
| Antithrombin | 3.8 ± 0.6** | 4.0 ± 0.7** | 2.3 ± 0.1** | 6.0 ± 0.3 |
| PBS | 7.9 ± 0.7 | 7.3 ± 0.8 | 7.3 ± 1.1 | 5.9 ± 0.9 |
| Endostatin | 3.6 ± 0.5** | 6.8 ± 0.8 | 2.6 ± 0.5** | 3.9 ± 0.6 |
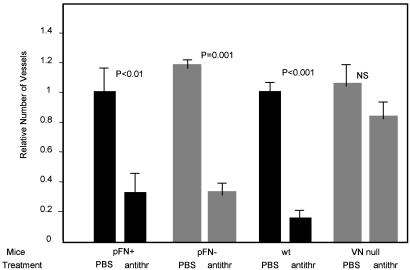
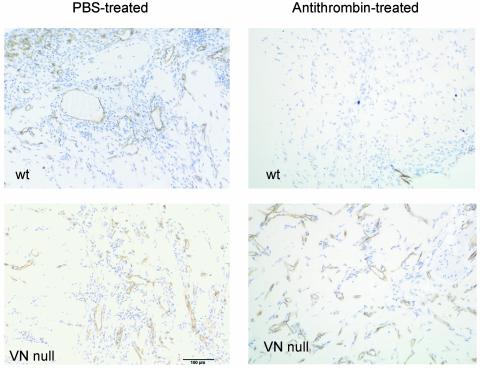
Antithrombin modified by denaturation or proteolysis becomes an angiogenesis inhibitor (5) and gains the ability to bind vitronectin (10, 11). Denatured antithrombin inhibited angiogenesis in wild-type mice and in the plasma fibronectin-deficient mice, but was inactive in the vitronectin-null mice. Blood vessel counts and hemoglobin values were highly significant (Fig. 3 and Table 1). Fig. 4 shows representative micrographs of matrigel plugs from the antithrombin-treated and control mice; these results show that vitronectin is required for the antiangiogenic activity of antithrombin.
Fig. 3.
Antithrombin is active in plasma fibronectin-deficient mice but is inactive in vitronectin-null mice. Mice with matrigel plugs were treated with seven daily i.p. injections of 270 μgor180 μg of antithrombin (antithr) or PBS, as in Fig. 2. The number of blood vessels in the plugs is shown. Results from mice treated with the two doses were pooled (16 mice in fibronectin experiments; 24 mice in vitronectin experiments).
Fig. 4.
Reduced blood vessel density in matrigel plugs from mice treated with antithrombin. Representative micrographs showing CD31-stained vessels in matrigel plugs of mice from four test groups in Fig. 3 are shown. (Magnification, ×400.)
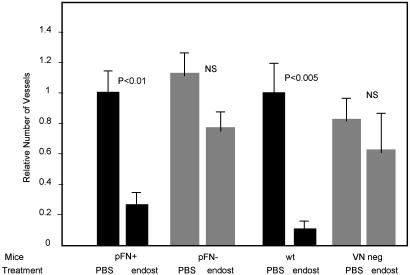
We also tested the dependence of the antiangiogenic activity of endostatin on plasma fibronectin and vitronectin. Like anastellin, endostatin is an angiogenesis inhibitor that is derived from an extracellular matrix protein. Endostatin was strongly antiangiogenic in plasma fibronectin-positive control mice and in wild-type mice, but its activity was impaired in fibronectin-deficient and vitronectin-null mice. The data suggested partial remaining activity in the mutant mice, but the difference to the PBS control was not statistically significant (Fig. 5). Thus, endostatin requires both fibronectin and vitronectin for full activity.
Fig. 5.
Endostatin antiangiogenic activity is impaired in plasma fibronectin-deficient and vitronectin-null mice. Mice with matrigel plugs were treated with seven daily i.p. injections of 120 μg of endostatin (endost) or PBS, as in Fig. 2. The number of blood vessels in the plugs from a total of 24 mice is shown.
Discussion
Our results suggest a common mechanism of action for protein inhibitors of angiogenesis: they form protein complexes with RGD-containing plasma adhesion proteins such as fibronectin or vitronectin. The binding specificity of anastellin for fibronectin (6, 9) and of modified antithrombin (5) for vitronectin (10, 11) was reflected in the dependence of their angiogenic activity on plasma fibronectin and vitronectin, respectively. That endostatin required both plasma fibronectin and vitronectin to be antiangiogenic in vivo was unexpected. Endostatin is not known to directly bind to fibronectin or vitronectin, but does bind to fibulin and nidogen (14). Fibulin, which is present in plasma at a concentration of ≈30 μg/ml (19), interacts with fibronectin (20). Endostatin (and anastellin and antithrombin) binds to heparin, and fibronectin has several heparin-binding sites (3, 5, 9, 21). Thus, one or more other proteins or proteoglycans could mediate a binding of endostatin to fibronectin and vitronectin in plasma or at cell surfaces. Angiostatin can bind vitronectin (13), as does at least one other antiangiogenic factor, the matricellular protein, SPARC (22). Thus, endostatin, angiostatin, and SPARC also have the potential of forming complexes with adhesion proteins in plasma.
The αvβ3, αvβ5, and α5β1 integrins, which are selectively expressed in angiogenic vessels (23, 24) are likely targets of the antiangiogenic protein/adhesion protein complexes. Both fibronectin and vitronectin are ligands for the αvβ3 integrin. Fibronectin also binds to α5β1, and vitronectin to αvβ5 (25). Endostatin binds to α5β1 (26) and tumstatin to αvβ3 (4), and these integrins mediate the in vitro effects of endostatin and tumstatin on endothelial cells (4, 27). Gene knockout experiments show that α5β1 is necessary for vascular development (28). The vasculature develops and angiogenesis takes place in mice that lack αvβ3 or all αv integrins (29, 30), but in an adult animal, perturbing the function of either α5β1 or αvβ3 causes endothelial cell apoptosis and inhibits angiogenesis (23, 24, 31). Moreover, synthetic RGD peptide polymers that mimic polymeric adhesion proteins can inhibit angiogenesis (32). The mechanism of endothelial damage by the antiangiogenic complexes remains to be elucidated. One possibility is that soluble complexes may elicit different integrin signals than the attachment of cells to a substrate, which promotes survival. Accumulation of RGD-containing peptides in the cytoplasm has been reported to initiate apoptosis (33, 34); internalization and proteolysis of antiangiogenic factor/adhesion protein complexes could provide a supply of cytoplasmic RGD peptides. Alternatively, the integrin-binding complexes could bind to circulating endothelial precursor cells (35), preventing them from contributing to the formation of new blood vessels. The hypothesis that RGD-directed integrins on angiogenic endothelial cells would be the common target of adhesion protein complexes assembled by the antiangiogenic proteins, provides a unifying explanation for the activities of a bewildering number of diverse antiangiogenic factors.
In addition to being relevant to the understanding of the control of angiogenesis, our results may allow antiangiogenic compounds to be used more effectively in the clinic. For example, some cancer patients who have received chemotherapy have low fibronectin levels (36). An antiangiogenic protein that depends on plasma fibronectin for its activity could be less effective in patients with low fibronectin levels. Little information is available on plasma vitronectin levels in pathological conditions, but similar considerations may apply to antithrombin and other angiogenesis inhibitors that might depend on vitronectin.
Acknowledgments
We thank Dr. David Loskutoff for the vitronectin-null mice and Dr. Eva Engvall, Maria Akerman, and Dr. Kristiina Vuori for comments on the manuscript. This work was supported by Department of Defense Grant DAMD17-00-1-0556, National Cancer Institute Grant CA88420, and Cancer Center Support Grant CA30199 (to E.R.), the Deutsche Forschungsgemeinschaft, and The Max Planck Society (R.F.).
Abbreviation: bFGF, basic fibroblast growth factor.
References
- 1.Good, D. J., Polverini, P. J., Rastinejad, F., Le Beau, M. M., Lemons, R. S., Frazier, W. A. & Bouck, N. P. (1990) Proc. Natl. Acad. Sci. USA 87, 6624–6628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.O'Reilly, M. S., Holmgren, L., Shing, Y., Chen, C., Rosenthal, R. A., Moses, M., Lane, W. S., Cao, Y., Sage, E. H. & Folkman, J. (1994) Cell 79, 315–328. [DOI] [PubMed] [Google Scholar]
- 3.O'Reilly, M. S., Boehm, T., Shing, Y., Fukai, N., Vasios, G., Lane, W. S., Flynn, E., Birkhead, J. R., Olsen, B. R. & Folkman, J. (1997) Cell 88, 277–285. [DOI] [PubMed] [Google Scholar]
- 4.Maeshima, Y., Sudhakar, A., Lively, J. C., Ueki, K., Kharbanda, S., Kahn, C. R., Sonenberg, N., Hynes, R. O. & Kalluri, R. (2002) Science 295, 140–143. [DOI] [PubMed] [Google Scholar]
- 5.O'Reilly, M. S., Pirie-Shepherd, S., Lane, W. S. & Folkman, J. (1999) Science 285, 1926–1928. [DOI] [PubMed] [Google Scholar]
- 6.Pasqualini, R., Bourdoulous, S., Koivunen, E., Woods, V. L., Jr., & Ruoslahti, E. (1996) Nat. Med. 2, 1197–1203. [DOI] [PubMed] [Google Scholar]
- 7.Yi, M. & Ruoslahti, E. (2001) Proc. Natl. Acad. Sci. USA 98, 620–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morla, A. & Ruoslahti, E. (1992) J. Cell Biol. 118, 421–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morla, A., Zhang, Z. & Ruoslahti, E. (1994) Nature 367, 193–196. [DOI] [PubMed] [Google Scholar]
- 10.Ill, C. R. & Ruoslahti, E. (1985) J. Biol. Chem. 260, 15610–15615. [PubMed] [Google Scholar]
- 11.de Boer, H. C., Preissner, K. T., Bouma, B. N. & de Groot, P. G. (1992) J. Biol. Chem. 267, 2264–2268. [PubMed] [Google Scholar]
- 12.Tomasini, B. R. & Mosher, D. F. (1991) Prog. Hemost. Thromb. 10, 269–305. [PubMed] [Google Scholar]
- 13.Kost, C., Benner, K., Stockmann, A., Linder, D. & Preissner, K. T. (1996) Eur. J. Biochem. 236, 682–688. [DOI] [PubMed] [Google Scholar]
- 14.Miosge, N., Sasaki, T. & Timpl, R. (1999) FASEB J. 13, 1743–1750. [DOI] [PubMed] [Google Scholar]
- 15.Fulgham, D. L., Widhalm, S. R., Martin, S. & Coffin, J. D. (1999) Endothelium 6, 185–195. [DOI] [PubMed] [Google Scholar]
- 16.Ngo, C. V., Gee, M., Akhtar, N., Yu, D., Volpert, O., Auerbach, R. & Thomas-Tikhonenko, A. (2000) Cell Growth Differ. 11, 201–210. [PMC free article] [PubMed] [Google Scholar]
- 17.Sakai, T., Johnson, K. J., Murozono, M., Sakai, K., Magnuson, M. A., Wieloch, T., Cronberg, T., Isshiki, A., Erickson, H. P. & Fassler, R. (2001) Nat. Med. 7, 324–330. [DOI] [PubMed] [Google Scholar]
- 18.Zheng, X., Saunders, T. L., Camper, S. A., Samuelson, L. C. & Ginsburg, D. (1995) Proc. Natl. Acad. Sci. USA 92, 12426–12430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Argraves, W. S., Tran, H., Burgess, W. H. & Dickerson, K. (1990) J. Cell Biol. 111, 3155–3164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Timpl, R., Sasaki, T., Kostka, G. & Chu, M. L. (2003) Nat. Rev. Mol. Cell Biol. 4, 479–489. [DOI] [PubMed] [Google Scholar]
- 21.Ruoslahti, E. (1988) Annu. Rev. Biochem. 57, 375–413. [DOI] [PubMed] [Google Scholar]
- 22.Chlenski, A., Liu, S., Crawford, S. E., Volpert, O. V., DeVries, G. H., Evangelista, A., Yang, Q., Salwen, H. R., Farrer, R., Bray, J. & Cohn, S. L. (2002) Cancer Res. 62, 7357–7363. [PubMed] [Google Scholar]
- 23.Brooks, P. C., Clark, R. A. & Cheresh, D. A. (1994) Science 264, 569–571. [DOI] [PubMed] [Google Scholar]
- 24.Kim, S., Bell, K., Mousa, S. A. & Varner, J. A. (2000) Am. J. Pathol. 156, 1345–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ruoslahti, E. (1996) Annu. Rev. Cell Dev. Biol. 12, 697–715. [DOI] [PubMed] [Google Scholar]
- 26.Rehn, M., Veikkola, T., Kukk-Valdre, E., Nakamura, H., Ilmonen, M., Lombardo, C., Pihlajaniemi, T., Alitalo, K. & Vuori, K. (2001) Proc. Natl. Acad. Sci. USA 98, 1024–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sudhakar, A., Sugimoto, H., Yang, C., Lively, J., Zeisberg, M. & Kalluri, R. (2003) Proc. Natl. Acad. Sci. USA 100, 4766–4771. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 28.Yang, J. T., Rayburn, H. & Hynes, R. O. (1993) Development (Cambridge, U.K.) 119, 1093–1105. [DOI] [PubMed] [Google Scholar]
- 29.Reynolds, L. E., Wyder, L., Lively, J. C., Taverna, D., Robinson, S. D., Huang, X., Sheppard, D., Hynes, R. O. & Hodivala-Dilke, K. M. (2002) Nat. Med. 8, 27–34. [DOI] [PubMed] [Google Scholar]
- 30.Hynes, R. O. (2002) Nat. Med. 8, 918–921. [DOI] [PubMed] [Google Scholar]
- 31.Cheresh, D. A. & Stupack, D. G. (2002) Nat. Med. 8, 193–194. [DOI] [PubMed] [Google Scholar]
- 32.Saiki, I., Murata, J., Makabe, T., Nishi, N., Tokura, S. & Azuma, I. (1990) Jpn. J. Cancer Res. 81, 668–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Buckley, C. D., Pilling, D., Henriquez, N. V., Parsonage, G., Threlfall, K., Scheel-Toellner, D., Simmons, D. L., Akbar, A. N., Lord, J. M. & Salmon, M. (1999) Nature 397, 534–539. [DOI] [PubMed] [Google Scholar]
- 34.Adderley, S. R. & Fitzgerald, D. J. (2000) J. Biol. Chem. 275, 5760–5766. [DOI] [PubMed] [Google Scholar]
- 35.Rafii, S., Lyden, D., Benezra, R., Hattori, K. & Heissig, B. (2002) Nat. Rev. Cancer 2, 826–835. [DOI] [PubMed] [Google Scholar]
- 36.Choate, J. J. & Mosher, D. F. (1983) Cancer 51, 1142–1147. [DOI] [PubMed] [Google Scholar]