Abstract
CD95 type I and II cells differ in their dependence on mitochondria to execute apoptosis, because antiapoptotic members of the Bcl-2 family render only type II cells resistant to death receptor-induced apoptosis. They can also be distinguished by a more efficient formation of the death-inducing signaling complex in type I cells. We have identified a soluble form of CD95 ligand (S2) that is cytotoxic to type II cells but does not kill type I cells. By testing 58 tumor cell lines of the National Cancer Institute's anticancer drug-screening panel for apoptosis sensitivity to S2 and performing death-inducing signaling complex analyses, we determined that half of the CD95-sensitive cells are type I and half are type II. Most of the type I cell lines fall into a distinct class of tumor cells expressing mesenchymal-like genes, whereas the type II cell lines preferentially express epithelium-like markers. This suggests that type I and II tumor cells represent different stages of carcinogenesis that resemble the epithelial–mesenchymal transition. We then screened the National Cancer Institute database of >42,000 compounds for reagents with patterns of growth inhibition that correlated with either type I or type II cell lines and found that actin-binding compounds selectively inhibited growth of type I cells, whereas tubulin-interacting compounds inhibited growth of type II cells. Our analysis reveals fundamental differences in programs of gene expression between type I and type II cells and could impact the way actin- and microtubule-disrupting antitumor agents are used in tumor therapy.
CD95 is a member of the family of the death receptors that initiate apoptosis by recruiting Fas-associated death domain protein (FADD), procaspase-8, procaspase-10, and cellular FLICE-like inhibitory protein to the death-inducing signaling complex (DISC), which forms after binding of the cognate ligand (CD95L) (1). CD95 type I and II cells differ in their dependence on mitochondria for the execution of apoptosis in that type II cells require mitochondrial amplification to die (2). Hence, overexpression of antiapoptotic Bcl-2 family members only inhibits CD95-mediated apoptosis in type II cells. We proposed this CD95 two-pathway model based on a study of four tumor cell lines (2, 3), and a number of transgenic and knockout mice have demonstrated that this distinction also applies to normal tissues (e.g., liver cells are type II, and T cells are type I) (reviewed in ref. 4). One of the most striking differences between type I and II cells lies in the way the CD95 signal is generated at the receptor level. An efficient formation of the DISC is observed only in type I cells, whereas in type II cells it is difficult to detect by Western blotting (2). We recently demonstrated that formation of the DISC in type I cells involves F-actin (5) and that the receptor internalizes in an actin- and caspase-8-dependent fashion only in type I cells (6). These data suggest that type I cells differ from type II cells in the way the CD95 signal is initiated. This difference in signal initiation could result in a difference in sensitivity to CD95 stimuli.
The cognate CD95L is expressed as both a membrane-bound (mCD95L) and soluble (sCD95L) form that is generated by metalloprotease cleavage of mCD95L (7, 8). As yet, no clear specific separate function has been assigned to sCD95L or mCD95L. Here we uncover a striking difference in the response of type I and II tumor cell lines to different forms of CD95L. We found that a preparation of soluble sCD95L (S2) (9) efficiently kills type II cells. In contrast, type I cells are resistant to this cytotoxic activity. S2 therefore represents a tool for identifying type I and II cells. We applied this tool to a collection of 58 tumor cell lines of various histologic origin [of the Developmental Therapeutics Program of the National Cancer Institute (NCI)] (10). These cell lines have been subjected to a comprehensive microarray analysis to determine their patterns of gene expression and were found to cluster into two very distinct classes of cells (epithelium-like and mesenchymal-like) that share expression of similar sets of genes (11). We have determined that 22 of these 58 cell lines are CD95 apoptosis-sensitive and have classified half of these sensitive cells as type I and half as type II based on their sensitivity to S2 stimulation and their ability to form a DISC. Ten of 11 of the cell lines that we classified as type I cells were found in the mesenchymal branch, whereas 9 of 11 of the type II cells were found in the epithelial branch. The type I/type II status of the cells was used to query the public NCI Developmental Therapeutics Program anticancer drugscreening database (which contains data on >42,000 compounds) for cells with patterns of activity that could distinguish between type I and II cells. We identified a selective sensitivity of type I cells to actin-binding reagents and a selective sensitivity of type II cells to tubulin-binding compounds. Our data suggest fundamental differences in programs of gene expression between type I and II cells that cause them to respond differently to CD95 stimulation, actin-disrupting reagents, and compounds that disrupt microtubules.
Materials and Methods
Cell Lines. The B lymphoblastoid cell line SKW6.4, the T cell lines H9, Jurkat (clone E6-1 and JA3), JurkatR (12), FADD- and caspase-8-deficient Jurkat cells (13, 14), CEM, 293T, COS, and CT26-mCD95L cells were cultured in RPMI medium 1640 supplemented with 10% FCS/2 mM glutamine/100 units/ml penicillin/100 μg/ml streptomycin and maintained in 5% CO2 at 37°C. Of the 60 human tumor cell lines of the NCI drug-screening program, 58 were cultured in the same medium with 5% FCS without addition of antibiotics.
Antibodies, Plasmids, and Reagents. Anti-APO-1 is an agonistic mAb (IgG3, κ) recognizing an epitope on the extracellular portion of CD95 (15). The neutralizing anti-CD95L antibody clone NOK-1 (used for flow cytometry), the clone G247-4 (used for Western blotting), and the metalloprotease inhibitor KB8301 were obtained from Pharmingen. The neutralizing anti-CD95 mAb ZB4 was from Upstate Biotechnology (Lake Placid, NY). All other chemicals used were of analytical grade and purchased from Sigma, Molecular Probes, or Calbiochem. Plasmids to produce the recombinant human sCD95L, S1 and S2, and leucine zipper-tagged CD95L (LzCD95L) were described elsewhere (9, 16).
Production of sCD95L. To produce S1 and S2 ligands, 293T cells were transiently transfected by using the calcium phosphate method. Briefly, 106 cells were plated on 10-cm dishes and transfected with 10 μg of the CD95L constructs: pEF-BOS-SIG–hFasL (amino acids 103–281, S1) or pEF-BOS–hFasL (amino acids 137–281, S2). Cells were incubated for 72 h at 37°C. Supernatants were harvested, centrifuged to remove cells and debris, and concentrated (10-fold) by using centrifugal concentrators (10-kDa molecular mass cutoff). sCD95L in cell lysates and concentrated supernatant was characterized and quantified by Western blotting using the anti-CD95L antibody clone G247-4. S2-containing supernatants were also generated by using COS cells for transient transfections, and the sCD95L preparations had similar activities (data not shown). Furthermore, to establish S2 as being the only apoptosis-inducing activity in these supernatants, we demonstrated that such a 293T/S2 supernatant selectively depleted of sCD95L by using the anti-CD95L mAb NOK-1 could not enhance FLAG-sCD95L or staurosporine-induced apoptosis on Jurkat cells (data not shown).
Induction of apoptosis, cytotoxicity assays, and CD95 and CD95L surface staining are described in Supporting Materials and Methods, which is published as supporting information on the PNAS web site, www.pnas.org.
Results
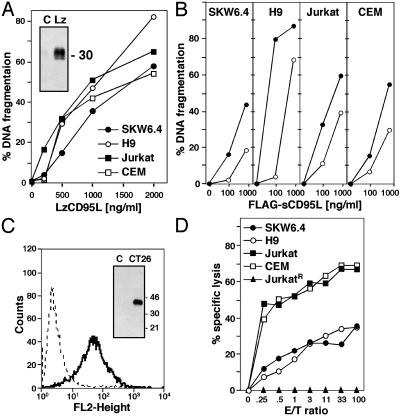
Sensitivity of Type I and II Cells to Different Aggregated Forms of CD95L. We previously postulated that a physiological difference between CD95 type I and II cells could be in response to different forms of the CD95L, sCD95L vs. mCD95L (17). To test this assumption we first determined the sensitivity of the four tumor cell lines we originally characterized (2) to highly aggregated LzCD95L. We did not find a significant difference in apoptosis sensitivity between these cell lines (Fig. 1A). The four cell lines were also similarly sensitive to FLAG-sCD95L, although H9 cells showed the highest sensitivity (Fig. 1B). Cytotoxicity of FLAG-sCD95L could be increased in all cells by adding crosslinking anti-FLAG antibody, consistent with a previous report (7). We did not observe a difference in apoptosis sensitivity between type I and II cells with these widely used CD95L preparations. We then tested the activity of the authentic mCD95L using the mouse colon carcinoma cell line CT26 expressing human mCD95L (18). To prevent the generation of sCD95L, the experiment was performed in the presence of the metalloprotease inhibitor KB8301, resulting in significant cell surface expression of mCD95L (Fig. 1C). No sCD95L was detected by Western blotting of total cell extracts of these cells (Fig. 1C). We cocultured the CT26 cells with the four cell lines loaded with 51Cr. In both a standard 4-h (data not shown) and 7-h (Fig. 1D) chromium-release assay the two type II cells were killed more efficiently than the two type I cells. Apoptosis was CD95-specific, because the CD95-negative JurkatR cells (12) were resistant to apoptosis in this assay. When we repeated the assay as a 20-h cytotoxicity assay, no significant difference between type I and II cells was detected (data not shown). These data suggest that mCD95L is more efficient in acute apoptosis induction in type II cells.
Fig. 1.
Sensitivity of type I and II cells to various forms of CD95L. (A) Apoptosis assay of cells treated for 16 h with LzCD95L. (Inset) A G247-4 anti-CD95L immunoblot of LzCD95L. (B) Apoptosis assay of cells treated for 16 h with FLAG-sCD95L in the absence (open circles) or presence (filled circles) of anti-FLAG antibodies. (C) Fluorescence-activated cell sorter analysis of CD95L expression on KB8301-treated CT26-mCD95L cells. Dotted line, isotype control; bold line, NOK-1 staining. (Inset) A G247-4 anti-CD95L immunoblot of mCD95L. (D) In vitro lysis of 51Cr-labeled type I and II cells by CT26-mCD95L cells. After 7 h of incubation, radioactivity in each well was measured and the percentage of specific lysis was calculated as described in Materials and Methods.
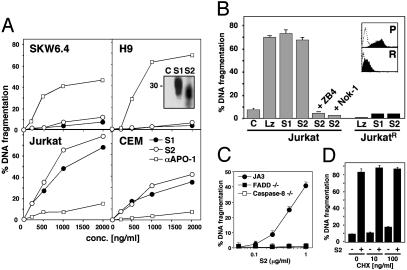
Differential Sensitivity of Type I and II Cells to sCD95L. The agonistic anti-CD95 mAb anti-APO-1 is an IgG3 antibody that aggregates through interactions between their constant regions (19). The apoptosis-inducing activity of this antibody varies depending on its aggregation state (unpublished observation). We tested a number of anti-APO-1 preparations and identified preparations with very low cytotoxicity on type II cells but high cytotoxicity on the two type I cells (Fig. 2A). The addition of very small amounts of protein A to anti-APO-1 (1 ng/ml protein A added to 1 μg/ml anti-APO-1) resulted in similar apoptosis sensitivity of all four cell lines (data not shown). We next used two secreted uncrosslinked forms of sCD95L that had been shown to be active on certain CD95 high-expressing cells but not on others (9). S1 contains the entire extracellular domain of CD95L (amino acids 103–281) and S2 only the trimerizing domain (amino acids 137–281). We transfected 293T cells with these sCD95L constructs and detected sCD95L at the expected molecular weight in the supernatant of these cells (Fig. 2 A Inset). We tested these CD95L preparations on the four cell lines. Surprisingly, both sCD95L preparations were highly cytotoxic to Jurkat and CEM cells but induced almost no apoptosis in SKW6.4 and H9 cells (Fig. 2 A). S1 and S2 therefore have cytotoxic activities that are inverted compared with anti-APO-1.
Fig. 2.
sCD95L efficiently kills type II, but not type I, cells. (A) Apoptosis assay of cells treated for 16 h with S1, S2, or anti-APO-1 (αAPO-1). (B) Apoptosis assay of cells incubated for 16 h with 1 μg/ml of control supernatant (C), LzCD95L (Lz), S1, or S2. In some cases S2 stimulation was performed in the presence of 1 μg/ml anti-CD95 (ZB4) or anti-CD95L (Nok-1) neutralizing antibodies. (Inset) Levels of surface CD95 on parental Jurkat (P) and JurkatR (R) cells as determined by flow cytometry. (C) Apoptosis assay of Jurkat mutants incubated for 16 h with S2. (D) Apoptosis assay of Jurkat cells pretreated with the indicated concentration of cycloheximide (CHX) and stimulated for 16 h with 1 μg/ml S2.
To ensure that the cytotoxic activity in our preparation was sCD95L and not another compound present in these concentrated supernatants, we tested the apoptotic activity of S1 and S2 on the JurkatR cells. In contrast to the parental cell line, apoptosis was not induced in the CD95 low-expressing Jurkat cells, JurkatR, after treatment with these ligands (Fig. 2B). Specificity of this apoptotic effect was also established by preincubation with either a neutralizing anti-CD95L (Nok-1 in Fig. 2B) or a neutralizing anti-CD95 (ZB4 in Fig. 2B) antibody. We therefore conclude that apoptosis induced by S2 is strictly dependent on CD95L–CD95 interaction. Furthermore, we found that S2 could not kill FADD- or caspase-8-deficient mutant Jurkat cells but killed the parental Jurkat cell line JA3 (Fig. 2C), suggesting that S2 induced the canonical apoptosis pathway in type II cells, which requires FADD and caspase-8. Because both S1 and S2 had similar activities, we performed all subsequent experiments with S2. To address the possibility that S2 did not induce apoptosis by directly activating the CD95 pathway but rather by causing transcriptional up-regulation of another cytotoxic factor which in turn killed the cells, we blocked protein synthesis by treating the cells with cycloheximide (Fig. 2C) followed by stimulation with sCD95L. At 100 ng/ml, cycloheximide was slightly cytotoxic to Jurkat cells but did not inhibit apoptosis induced through CD95 by the addition of S2 (Fig. 2C). In summary, CD95L preparations of artificially crosslinked CD95L (LzCD95L) or FLAG-sCD95L plus anti-FLAG antibody were able to kill all cells equally. In contrast, both mCD95L and sCD95L preparations were more potent in inducing apoptosis in type II cells. The effect was most pronounced with S1 and S2, which did not induce apoptosis in type I cells.
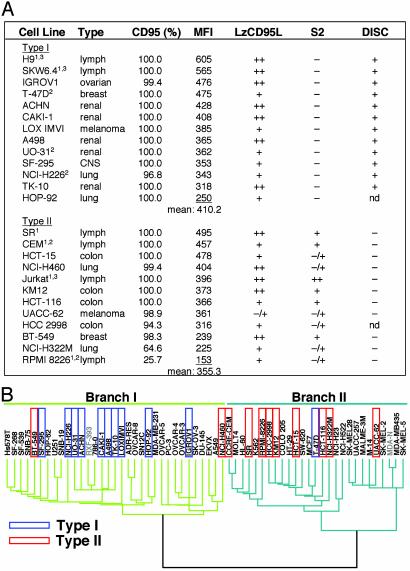
CD95 Type I and II Tumor Cells Fall into Two Distinct Classes with Fundamental Differences in Programs of Gene Expression. To test whether the finding with the four prototype type I and II cells could be applied to cancer cell lines in general, we tested 58 tumor cell lines of the drug-screening panel of the NCI (NCI60). Twenty-two of these cell lines were consistently sensitive to apoptosis induction through CD95 when incubated with anti-APO-1 antibody or LzCD95L, whereas 23 cell lines were completely resistant to CD95-mediated apoptosis in all experiments and all assays used (Fig. 3A and data not shown). Consistent with our previous observations, we did not find a correlation between the surface expression of CD95 and the CD95 apoptosis sensitivity (data not shown). Eleven of the NCI60 cells that were sensitive to LzCD95L were resistant to S2. The remaining cells showed moderate or high sensitivity to S2 (Fig. 3A). We tentatively classified the latter cell lines as type II. We have shown previously that type II, in contrast to type I, cells form a DISC at very low levels, resulting in highly reduced generation of active caspase-8 at the activated receptor, thus providing an explanation for the need for mitochondrial amplification to execute apoptosis in type II cells (2, 3). We therefore tested whether this property of type I and II cells was also found in the cell lines we had tentatively classified as type I or II. Most of the CD95-sensitive cell lines tested in this study were subjected to an analysis of DISC formation. All cell lines tested that were resistant to S2 efficiently formed a DISC, whereas FADD or caspase-8 recruitment to the DISC was not detected by Western blotting in any of the tested S2-sensitive cells (Fig. 3A). These results confirm that cells that are insensitive to the cytotoxic effects of S2 behave like type I cells, whereas cells that are sensitive to S2 behave like type II cells.
Fig. 3.
Type I and II tumor cell lines among the NCI60 cells fall into two major classes that differ by expressing different sets of genes. (A) Cell lines were stained for CD95 surface expression. Shown is the percentage of CD95 surface expression, the mean fluorescence intensity (MFI), and apoptosis sensitivity as measured after incubation with 1 μg/ml of either LzCD95L or S2 for 20 h. Cell lines are ordered according to the mean fluorescence intensity of CD95 surface expression. Apoptosis sensitivity was determined by three different methods. (i) Morphological changes typical for apoptosis such as membrane blebbing and cell detachment (only adherent cells). All cell lines were tested at least three times. (ii) The 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) assay (at least three assays per cell line). (iii) An analysis of formation of the DISC. 1, all lymphoid cells were additionally tested for DNA degradation by using propidium iodide staining of nuclei as described in Materials and Methods; 2, these cell lines were more sensitive (++) to anti-APO-1 plus protein A; 3, not part of the NCI drug-screening panel. The tumor origin is given. nd, not determined; –, apoptotic cells <10%; –/+, 10–25%; +, 25–50%; ++, >50%. The cell lines 786-0, A549/ATCC, COLO 205, DU-145, EKVX, HS 578T, HT29, K562, M14, MALME-3M, MCF-7, MDA-MB-231, MDA-MB-435, MOLT4, NCI/ADR-RES, OVCAR-5, SF-268, SF-539, SK-MEL-2, SK-MEL-5, SK-OV-3, SN12C, SNB-19, SW-620, UACC-257, U251, and HOP-62 were completely and consistently resistant to CD95-mediated apoptosis in all assays (data not shown). (B) CD95-sensitive tumor cell lines identified as types I and II as shown in A are boxed in blue and red, respectively. The length of the dendrogram branches connecting pairs of nodes is directly proportional to the differences in gene expression of the 1,161 transcripts (of 9,703 total) that had been shown to vary at least 7-fold among the NCI60 cells (11). Cell lines in gray were not available to us for analysis. The two main branches representing tumor cells with increased expression of either mesenchymal (Branch I) or epithelial (Branch II) markers are colored in green or turquoise, respectively. [Reproduced with permission from Ross et al. (11) (Copyright 2000, Nature Genetics).]
The NCI60 cells have been segregated into clusters by determining the expression levels of 8,000 distinct human transcripts (11). In addition to the expected expression pattern related to the histologic origin of tumor cell lines (e.g., all renal carcinoma cell lines were found in one cluster), Ross et al. (11) identified a dividing difference between these tumor cell lines that allowed the cells to be grouped into two major branches: branch I contained a number of cell lines expressing genes characteristic of mesenchymal/stromal-derived cells, whereas branch II contained many cell lines that express genetic markers characteristic of epithelial cells (Fig. 3B). However, a number of cell lines cosegregated with these branches without having characteristics of mesenchymal or epithelial cells (e.g., leukemic cell lines were found in branch II). When we compared the results of our analysis of these cells with the differences in these cells derived from the gene screen, we observed that most of the cell lines we determined to be type I were found in the mesenchymal branch I (10 of 11, P = 0.001) of the dendrogram (Fig. 3B), whereas type II cell lines were predominantly found in the epithelial branch II (9 of 11, P = 0.026). Carcinogenesis has been proposed to be similar to the epithelial–mesenchymal transition (EMT) found during development of many tissues (20). Type I and II tumor cells therefore most likely represent different stages of carcinogenesis from a highly differentiated epithelial to a dedifferentiated mesenchymal stage.
Differences in Gene Expression Between Type I and II Cells. Because of the correlation between the CD95 apoptosis cell type with the two branches defined by expression of different classes of genes, we were interested in whether we would find such differences between type I and II cells. Using the information on the 22 typed NCI60 cells we performed a compare analysis (21) of the data against the gene array results on the 8,000 distinct human genes that had been performed with these cell lines (11) (see Fig. 4, which is published as supporting information on the PNAS web site). CD95 was found to be expressed at higher levels in type I cells, consistent with a somewhat higher CD95 protein expression in type I cells (see mean fluorescence intensities in Fig. 3A). Among other genes with expression that tended to be higher in type I cells were a number of actin-binding or -regulating genes, consistent with our recent finding that type I cells require F-actin to efficiently signal CD95-mediated apoptosis, cluster, and internalize CD95 (6). Other groups of genes with expression that correlated with type I cells were genes most typically expressed in mesenchymal cells, such as integrins, collagens, LIM domain-containing proteins, and angiogenic factors. Furthermore, fibroblast growth factor receptor 1 was overrepresented. Interestingly, fibroblast growth factor receptor 1 signaling has been shown to trigger the EMT (22). The genes expressed in type II cells belonged to diverse functional groups.
Inverted Responses of Type I and II Cells to Actin- and Tubulin-Binding Compounds. The differences between type I and II cells in the expression of multiple genes suggested different sensitivity to antitumor reagents. To identify compounds that selectively target type I or II cells, we performed a compare analysis (21) of the anticancer drug-screen database at the NCI containing information on >42,000 compounds including most of the standard antitumor drugs (see http://dtp.nci.nih.gov/index.html). Data on both total growth inhibition and 50% growth inhibition as endpoints were searched (a complete list of results can be found in Fig. 5, which is published as supporting information on the PNAS web site). At the total growth inhibition endpoint, most of the compounds with the highest correlations with type I cells were actin-binding or -disrupting agents, indicating that type I cells are more sensitive to growth inhibition by these compounds than type II cells. In fact, of the 20 most effective compounds that inhibited the growth of type I cells, 12 are known actin-binding or F-actin-disrupting reagents. A total of 16 such compounds within the top 58 drugs with Pearson correlation coefficients (PCCs) of >0.5 were found to preferentially inhibit the growth of type I cells. Results for five of these actin-selective compounds are shown in the left half of Table 1. These correlations were quite high; e.g., Act1 (NSC 112167) had a PCC of 0.894, where a PCC of 1 denotes a perfect match. This is highly significant, with a P value of 0.00047 (after applying the Bonferroni adjustment for multiple comparisons). No actin-binding compounds were found with high correlations for type II cells. At the 50% growth inhibition endpoint, the highest correlations with type II cells were found with a number of tubulin-directed agents (or structural analogs of known tubulin binders), tubulin stabilizers as well as tubulin disrupters. These data indicate that type II cells are more sensitive to tubulin-binding compounds. Five of the 18 tubulin-binding compounds identified are listed in the right half of Table 1. Both the actin- and tubulin-directed compounds were fairly potent in the 60-cell-line screen, with mean total growth inhibition/50% growth inhibition values in the micromolar to nanomolar range. All these compounds exhibited differential activity among the 22 cell lines, with the most sensitive cell lines requiring ≈100- to 10,000-fold lower concentrations to reach each endpoint than the least sensitive cell lines (for individual results of all actin- and tubulin-binding compounds on all 22 cell lines see Fig. 6, which is published as supporting information on the PNAS web site). These results demonstrate that CD95 type I and II cells can be distinguished on the basis of their sensitivity to growth inhibition by actin- or tubulin-binding compounds. Among these compounds, many have either been considered for use or are already in use to treat cancer, such as the β-tubulin-binding reagent paclitaxel (Taxol).
Table 1. Compounds that disrupt microfilaments or microtubules selectively target type I and type II cells, respectively.
| Act1 | Act2 | Act3 | Act4 | Act5 | Tub1 | Tub2 | Tub3 | Tub4 | Tub5 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Cell line | ||||||||||
| Type I | ||||||||||
| IGROV1 | + | + | + | + | + | + | o | o | + | - |
| T-47D | nd | nd | nd | - | nd | - | + | - | - | - |
| ACHN | + | + | - | + | + | - | - | - | - | - |
| CAKI-1 | + | + | + | + | + | - | - | - | - | - |
| LOX IMVI | + | + | + | - | + | + | - | + | o | o |
| A498 | + | + | + | + | + | - | - | - | - | - |
| UO-31 | + | + | + | + | + | - | - | - | - | - |
| SF-295 | + | + | + | - | o | - | - | + | - | - |
| NCI-H226 | + | + | + | + | + | - | - | - | - | o |
| TK-10 | + | + | + | + | + | - | - | - | - | - |
| HOP-92 | + | + | + | + | + | - | - | - | - | - |
| Type II | ||||||||||
| SR | - | - | - | - | + | + | - | + | + | + |
| CEM | - | - | + | - | - | + | + | + | + | + |
| HCT-15 | - | - | - | - | - | - | - | + | - | + |
| NCI-H460 | - | - | - | - | - | + | + | + | + | + |
| KM12 | - | - | - | - | - | + | + | + | + | - |
| HCT-116 | - | - | - | - | - | + | + | + | + | + |
| UACC-62 | - | - | - | - | - | + | o | + | + | + |
| HCC 2998 | - | - | + | - | - | + | + | + | + | - |
| BT-549 | nd | nd | nd | o | nd | + | + | + | + | + |
| NCI-H322M | - | - | - | - | - | + | nd | + | + | - |
| RPMI 8226 | - | - | o | - | - | + | + | + | + | + |
| PCC | 0.89 | 0.85 | 0.82 | 0.80 | 0.80 | -0.69 | -0.67 | -0.67 | -0.63 | -0.62 |
| P (× 10-5) | 0.011 | 0.18 | 1.0 | 0.89 | 3.0 | 82 | 180 | 71 | 310 | 230 |
| M conc., μM | 2.42 | 4.71 | 22.64 | 6.19 | 1.13 | 0.107 | 3.85 | 0.07 | 0.57 | 2.81 |
| Δ conc., logs | 1.4 | 2.4 | 1.4 | 2.6 | 3.4 | 2.0 | 2.3 | 3.7 | 2.5 | 2.0 |
The information on CD95 cell type of the NCI60 cell lines (Fig. 3A) was used in a compare analysis against the NCI anticancer drug-screen database of >42,000 compounds to identify compounds with patterns of growth inhibition that correlated with either type I or type II cells. Results for five of the actin-binding compounds (Act 1-5) that came up in the search with total growth inhibition as endpoint and results for five of the tubulin-binding compounds (Tub 1-5) that came up in the search with 50% growth inhibition as endpoint are shown. Cell lines that were less sensitive than the mean (across all 22 cell lines) are denoted by a minus (-), and cell lines that were more sensitive than the mean are designated by a plus (+). Cell lines not differing from the mean are indicated by an o. nd, not determined. Act1, NSC 112167, cucurbitacin I; Act2, NSC 94743, cucurbitacin A; Act3, NSC 112166, cucurbitacin K; Act4, NSC 681481, jasplakinolide analog; Act5, NSC 606195, dolastatin 11; Tub1, NSC 666608, Taxol analog; Tub2, NSC 658831, Taxol analog; Tub3, NSC 650773, combretastatin analog; Tub4, NSC 666606, Taxol analog; Tub5, NSC 659853, 2-methoxyestradiol. P values (two-tailed) were not corrected for numbers of compounds in the database. M conc., mean of effective concentration; Δ conc., range in concentration between the least and most sensitive cell line. Complete 60-cell-line data for these compounds can be found at http://dtp.nci.nih.gov.
The Inverted Sensitivity of Type I and II Cells to Microfilament- and Microtubule-Disrupting Compounds Depends on a Functional CD95 Apoptosis Signaling Pathway. We found an almost complete correlation between the CD95 apoptosis cell type and the two branches of different gene-expression patterns. To test whether the differential sensitivity of tumor cell lines to actin- and tubulin-binding compounds depended only on whether cells are more epithelial- or mesenchymal-like and less dependent on whether they are CD95 type I or II cells, we performed a compare analysis of all cell lines we had determined to be resistant to CD95-mediated apoptosis. The results, shown in Fig. 7A, which is published as supporting information on the PNAS web site, demonstrate that only 8 (21.1%) of these 38 cell lines showed a differential sensitivity to either actin-disrupting compounds (tentatively grouped as type I cells) or microtubuleinteracting compounds (tentatively grouped as type II cells). In contrast, 16 (42.2%) of these cells did not show a differential sensitivity to these two classes of reagents, and eight cell lines had not been tested for most of the actin-binding compounds and therefore could not be grouped. This result suggested that the exclusive sensitivity of tumor cell lines to either actin- or tubulin-binding compounds requires a functional CD95 signaling pathway. Further compare analyses (Figs. 8 and 9, which are published as supporting information on the PNAS web site) confirmed that, although there is overlap between the epithelial and mesenchymal groups of tumor cell lines with the type I and II groups, the mutually exclusive sensitivity of cell lines to actinand tubulin-binding compounds depends on a functional CD95 signaling pathway, and the tubulin-binding compounds in particular allow differentiation between type I and II cells.
Discussion
This study was undertaken to characterize CD95 type I and II cells in their response to different CD95-specific stimuli to eventually determine the physiological role of the type I and II distinction in tumor cells and normal tissues. We identified a form of sCD95L (S2) that selectively kills type II; type I cells were resistant to S2. This sCD95L was therefore used to type 58 of the NCI60 cells that have been characterized intensively (see http://dtp.nci.nih.gov). We found that 22 of these cell lines (38%) were moderately or highly sensitive to CD95-mediated apoptosis when stimulated with either LzCD95L (Fig. 3A) or crosslinked anti-APO-1 (data not shown). Of these 22 cell lines, 11 were sensitive to S2, whereas the remaining 11 cell lines were completely resistant to S2 (Fig. 3A). Efficient DISC formation could be detected only in the cell lines that were resistant to S2, validating S2 as a tool to differentiate type I and II cells. A comparison of our results with a gene-array analysis previously performed on these cells (11) revealed that type I and II cells represent two classes of cells that express different sets of genes, suggesting that the differential expression of multiple genes determines whether a CD95-sensitive cell dies through a mitochondrial-dependent or -independent pathway.
mCD95L has been shown to be an apoptosis-inducing ligand, whereas sCD95L was proposed to be an antagonist to mCD95L (7, 8). This view changed recently with experiments that demonstrated that sCD95L gains activity similar to that of mCD95L when bound to extracellular matrix, a situation which is likely to be relevant in vivo (18). Nagata and coworkers (9) reported that a secreted form of sCD95L, S2, which was used in our study, was an active cytotoxic ligand that selectively killed only certain cells, such as mouse W4 cells. The interpretation of this observation was that this form of CD95L was cytotoxic only on very highly CD95-positive cells. Furthermore, it has been suggested that the reason for the reduced apoptosis sensitivity of type II cells could be a lower expression level of CD95 compared with type I cells (23). Our data now demonstrate that CD95 expression levels alone cannot account for the difference in apoptosis sensitivity of type I and II cells to different CD95-specific stimuli. The two type I cells (SKW6.4 and H9) we initially characterized (2) express significantly more CD95 than the two type II cells (Jurkat and CEM) (Fig. 3A) and are very sensitive to induction of apoptosis by crosslinked agonistic anti-CD95 antibodies or highly aggregated CD95Ls yet are resistant to S2. This confirms that S2 cytotoxicity depends on the cell type and not the level of CD95 expression, because it selectively kills type II, but not type I, cells. S2 could also induce apoptosis in type I cells when treated with cycloheximide, indicating that S2 bound to CD95 on type I cells and that it can induce apoptosis under certain circumstances (unpublished data).
The significant correlation between the CD95 cell type and the two distinct branches of tumor cells identified by Ross et al. (11) was surprising, considering the genomic instability of many tumor cell lines, and suggests that type I and II cells stably differ in the expression levels of hundreds of different genes. Cells in branch I (type I) preferentially express a number of mesenchymal markers, whereas cells in branch II (type II) express a number of epithelial markers. The major differences between branches I and II are likely a reflection of different stages of tumor development.
Changes during carcinogenesis are similar to the changes that occur during the development of certain tissues during embryogenesis. This process is referred to as the EMT (20). The EMT induces major changes in cell morphology and cell–cell contacts involving reorganization of the cytoskeleton including the microfilament system. The membrane-proximal signaling pathways involved in this transition have been described partially (20). Cells receive a signal through tyrosine kinase receptors such as fibroblast growth factor receptor 1 (22), which is followed by activation of the small GTPases Ras/Rho and Rac. Ras can activate phosphatidylinositol 3-kinase, which in turn can activate Rho and Rac. Activation of Rho/Rac causes reorganization of the actin cytoskeleton and activation of myelin light chain kinase. Interestingly many of the components of this signaling pathway were found to be upregulated in type I cells (highlighted in Fig. 4A). The gene product with the highest correlation with the type I group was the p85 regulatory subunit of the phosphatidylinositol 3-kinase (PCC, 0.774). Activation of phosphatidylinositol 3-kinase has been directly linked to the EMT (24). The second-best correlating gene was a homolog of a myosin light chain kinase (PCC, 0.773). Fibroblast growth factor receptor 1 was detected with a PCC of 0.734 and a Rho GTPase-activating protein with a PCC of 0.643. Furthermore, actin γ1 (PCC, 0.669), actin α1 (PCC, 0.551), and the actin-binding proteins tropomyosin 1 (PCC, 0.723) and myosin light chain (PCC, 0.643) were detected. Our results suggest that, during carcinogenesis, tumors develop from type II to type I. Future experiments may address whether such a rewiring of the CD95 signaling pathway also occurs during the EMT in vivo.
Our data strongly point to a link between actin and CD95 signaling in type I cells: (i) internalization and clustering of CD95 is only found in type I cells (6); (ii) type I cells have increased sensitivity to growth inhibition by a variety of actin-disrupting compounds; and (iii) a group of actin-regulating genes was found to be overrepresented in type I cells. Furthermore, our analysis has revealed a connection between the CD95 sensitivity status of cells and their sensitivity to actin disruption. This finding is consistent with a recent report that demonstrated that induction of apoptosis by actin disruption depends on expression of CD95, because cells from lpr (lymphoproliferative) mice, which have defective expression of CD95, were less sensitive to apoptosis induced by treatment with cytochalasin B (25).
In addition to solidifying the connection between actin and CD95 signaling in type I cells, our data uncovered an unexpected link between CD95 signaling in type II cells and microtubules, because CD95-sensitive type II cells were found to be more sensitive to growth inhibition induced by tubulin-binding or microtubule-disrupting compounds than type I cells. It is interesting to note that resistance of tumor cells to Taxol-induced apoptosis was shown recently to be caused by up-regulated ErbB2 expression through inhibition of p34cdc2 activation (26). Searching the NCI database for expression of proteins that have been studied in the NCI60 cells revealed ErbB2 as a protein with a high correlation of expression (PCC, 0.72) in type I cells (MT1174 at http://dtp.nci.nih.gov/mtargets/mt_index.html).
Our results may affect the strategy for antitumor drug use. Actin-disrupting drugs are currently being considered for use in tumor therapy, and tubulin-disrupting drugs such as paclitaxel (Taxol) and related compounds most likely represent the single most widely used and effective class of antitumor drugs in tumor therapy. Our data predict that actin-disrupting drugs should be more effective on tumors that can be classified as type I, whereas tubulin-disrupting drugs should be relatively less active in killing type I tumors but more effective on tumor cells that have been classified as type II.
Supplementary Material
Acknowledgments
We thank Drs. P. Krammer and H. Walczak (German Cancer Research Center, Heidelberg) for providing anti-APO-1 and LzCD95L, respectively. The CT26-CD95L cells and the constructs to express human sCD95L were kindly provided by Drs. G. Nabel (National Institutes of Health, Bethesda) and S. Nagata (Osaka University, Osaka), respectively. We also thank J. Blenis (Harvard Medical School, Boston) for the FADD- and caspase-8-deficient Jurkat cells and D. Scudiero of the NCI for providing the 58 tumor cell lines of the drug-screening panel. This work was funded by National Institutes of Health Grant GM61712. A.A.-S. was supported by Cancer Biology Training Program 5T32CA09594.
Abbreviations: FADD, Fas-associated death domain protein; DISC, death-inducing signaling complex; CD95L, CD95 ligand; mCD95L, membrane-bound CD95L; sCD95L, soluble CD95L; NCI, National Cancer Institute; LzCD95L, leucine zipper-tagged CD95L; EMT, epithelial–mesenchymal transition; PCC, Pearson correlation coefficient.
References
- 1.Peter, M. E. & Krammer, P. H. (2003) Cell Death Differ. 10, 26–35. [DOI] [PubMed] [Google Scholar]
- 2.Scaffidi, C., Fulda, S., Srinivasan, A., Friesen, C., Li, F., Tomaselli, K. J., Debatin, K. M., Krammer, P. H. & Peter, M. E. (1998) EMBO J. 17, 1675–1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scaffidi, C., Schmitz, I., Zha, J., Korsmeyer, S. J., Krammer, P. H. & Peter, M. E. (1999) J. Biol. Chem. 274, 22532–22538. [DOI] [PubMed] [Google Scholar]
- 4.Barnhart, B. C., Alappat, E. & Peter, M. E. (2003) Semin. Immunol. 15, 185–193. [DOI] [PubMed] [Google Scholar]
- 5.Algeciras-Schimnich, A., Shen, L., Barnhart, B. C., Murmann, A. E., Burkhardt, J. & Peter, M. E. (2002) Mol. Cell. Biol. 22, 207–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Algeciras-Schimnich, A. & Peter, M. E. (2003) FEBS Lett. 546, 185–188. [DOI] [PubMed] [Google Scholar]
- 7.Schneider, P., Holler, N., Bodmer, J. L., Hahne, M., Frei, K., Fontana, A. & Tschopp, J. (1998) J. Exp. Med. 187, 1205–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tanaka, M., Itai, T., Adachi, M. & Nagata, S. (1998) Nat. Med. 4, 31–36. [DOI] [PubMed] [Google Scholar]
- 9.Tanaka, M., Suda, T., Takahashi, T. & Nagata, S. (1995) EMBO J. 14, 1129–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stinson, S. F., Alley, M. C., Kopp, W. C., Fiebig, H. H., Mullendore, L. A., Pittman, A. F., Kenney, S., Keller, J. & Boyd, M. R. (1992) Anticancer Res. 12, 1035–1053. [PubMed] [Google Scholar]
- 11.Ross, D. T., Scherf, U., Eisen, M. B., Perou, C. M., Rees, C., Spellman, P., Iyer, V., Jeffrey, S. S., Van de Rijn, M., Waltham, M., et al. (2000) Nat. Genet. 24, 227–235. [DOI] [PubMed] [Google Scholar]
- 12.Peter, M. E., Dhein, J., Ehret, A., Hellbardt, S., Walczak, H., Moldenhauer, G. & Krammer, P. H. (1995) Int. Immunol. 7, 1873–1877. [DOI] [PubMed] [Google Scholar]
- 13.Juo, P., Woo, M. S., Kuo, C. J., Signorelli, P., Biemann, H. P., Hannun, Y. A. & Blenis J. (1999) Cell Growth Differ. 10, 797–804. [PubMed] [Google Scholar]
- 14.Juo, P., Kuo, C. J., Yuan, J. & Blenis, J. (1998) Curr. Biol. 8, 1001–1008. [DOI] [PubMed] [Google Scholar]
- 15.Trauth, B. C., Klas, C., Peters, A. M., Matzku, S., Möller, P., Falk, W., Debatin, K. M. & Krammer, P. H. (1989) Science 245, 301–305. [DOI] [PubMed] [Google Scholar]
- 16.Walczak, H., Degli-Esposti, M. A., Johnson, R. S., Smolak, P. J., Waugh, J. Y., Boiani, N., Timour, M. S., Gerhart, M. J., Schooley, K. A., Smith, C. A., et al. (1997) EMBO J. 16, 5386–5397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schmitz, I., Walczak, H., Krammer, P. H. & Peter, M. E. (2000) Cell Death Differ. 7, 756–758. [DOI] [PubMed] [Google Scholar]
- 18.Aoki, K., Kurooka, M., Chen, J. J., Petryniak, J., Nabel, E. G. & Nabel, G. J. (2001) Nat. Immunol. 2, 333–337. [DOI] [PubMed] [Google Scholar]
- 19.Dhein, J., Daniel, P. T., Trauth, B. C., Oehm, A., Moller, P. & Krammer, P. H. (1992) J. Immunol. 149, 3166–3173. [PubMed] [Google Scholar]
- 20.Boyer, B., Valles, A. M. & Edme, N. (2000) Biochem. Pharmacol. 60, 1091–1099. [DOI] [PubMed] [Google Scholar]
- 21.Paull, K. D., Shoemaker, R. H., Hodes, L., Monks, A., Scudiero, D. A., Rubinstein, L., Plowman, J. & Boyd, M. R. (1989) J. Natl. Cancer Inst. 81, 1088–1092. [DOI] [PubMed] [Google Scholar]
- 22.Ciruna, B. & Rossant, J. (2001) Dev. Cell 1, 37–49. [DOI] [PubMed] [Google Scholar]
- 23.Huang, D. C., Tschopp, J. & Strasser, A. (2000) Cell Death Differ. 7, 754–755. [DOI] [PubMed] [Google Scholar]
- 24.Bakin, A. V., Tomlinson, A. K., Bhowmick, N. A., Moses, H. L. & Arteaga, C. L. (2000) J. Biol. Chem. 275, 36803–36810. [DOI] [PubMed] [Google Scholar]
- 25.Kulms, D., Dussmann, H., Poppelmann, B., Stander, S., Schwarz, A. & Schwarz, T. (2002) Cell Death Differ. 9, 598–608. [DOI] [PubMed] [Google Scholar]
- 26.Tan, M., Jing, T., Lan, K. H., Neal, C. L., Li, P., Lee, S., Fang, D., Nagata, Y., Liu, J., Arlinghaus, R., et al. (2002) Mol. Cell 9, 993–1004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.