Abstract
National parks and bioreserves are key conservation tools used to protect species and their habitats within the confines of fixed political boundaries. This inflexibility may be their “Achilles' heel” as conservation tools in the face of emerging global-scale environmental problems such as climate change. Global climate change, brought about by rising levels of greenhouse gases, threatens to alter the geographic distribution of many habitats and their component species. With these changes comes great uncertainty about the future ability of parks and protected areas to meet their conservation mandates. We report here on an analysis aimed at assessing the extent of mammalian species turnover that may be experienced in eight selected U.S. national parks if climate change causes mammalian species within the continental U.S. to relocate to new geographic locations. Due to species losses of up to 20% and drastic influxes of new species, national parks are not likely to meet their mandate of protecting current biodiversity within park boundaries. This approach represents a conservative prognosis. As species assemblages change, new interactions between species may lead to less predictable indirect effects of climate change, increasing the toll beyond that found in this study.
Environmental managers are faced with the significant challenge of protecting species in the face of changing climate. This challenge is particularly formidable because species conservation is generally associated with protection strategies linked to particular pieces of property such as parks. National parks are used by nations around the earth and are increasingly called on to serve critical roles in species protection (1, 2). However, if global climate change alters the geographic distribution of habitats and wildlife species, the ability of parks to retain and protect species in the face of climate change is highly uncertain (3–9). We report here on an investigation aimed at assessing the ability of U.S. national parks to protect current mammalian diversity under a doubling of atmospheric carbon dioxide.
Recent empirical studies strongly suggest that wildlife species are already responding to recent global warming trends with significant shifts in range distribution (generally northward) and phenology (e.g., earlier breeding, flowering, and migration). Several recent reviews and metaanalyses provide a synthesis of contemporary global warming effects on wildlife (4–8). These empirical observations provide important evidence for the current response of wildlife to climate change; however, using the predictive power of models is essential for anticipating the large-scale and long-term effects of climate change as entire complex communities shift. Here we model wildlife distributional shifts that are likely to occur on a continental scale as vegetation responds to global warming and assess implications for the future role of the U.S. National Park System.
Methods
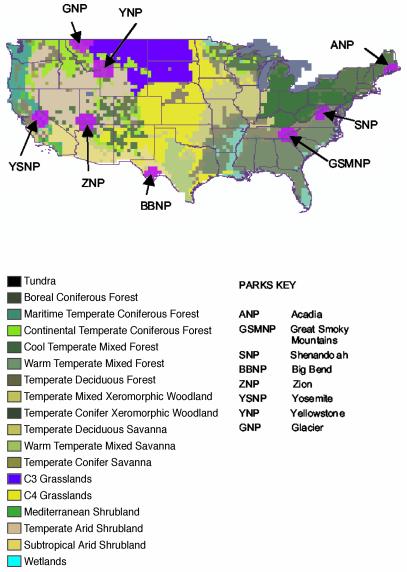
Current models of global climate change indicate that eastern and western ecosystems within the U.S. will be impacted differentially (3, 10). We therefore stratified the U.S. into eastern and western ecoregions (10) (divided by the Mississippi River) to ensure equitable representation of eastern and western parks. We then chose the following eight U.S. national parks from the larger pool of parks within those regions (Fig. 1): Acadia, Big Bend, Glacier, Great Smoky Mountains (GSM), Shenandoah, Yellowstone, Yosemite, and Zion. Our choice of specific national park was constrained by the geographic extent of climate change predictions [i.e., continental U.S. (3)], the regional availability of parks (e.g., there are absolutely more parks in western than eastern U.S.) and, crucially, by the availability of detailed mammalian species lists for each park.
Fig. 1.
Geographic distribution of major vegetation zones found across the continental U.S., with selected national parks and their greater surrounding ecosystems indicated in magenta. The map is reproduced in a gis by using data provided by the VEMAP Project (3).
Data Sets. We assembled data on current distributions of 213 mammalian species (132 species currently inhabiting at least one of the eight selected parks and 81 additional species available from the Faunmap database, (www.museum.state.il.us/research/faunmap) representing the major taxonomic orders within the continental U.S. (including Artiodactyla, Carnivora, Chiroptera, Insectivora, Lagomorpha, and Rodentia). Current distribution data for U.S. mammalian species were obtained from the Faunmap Project. We also obtained a database of the location of ecosystem types currently found within the continental U.S. and those expected under a doubling of atmospheric CO2. Current and predicted future distributions of ecosystem types [assuming CGCM2 GCM atmospheric conditions and coupled to the Mapped Atmosphere–Plant–Soil System (MAPSS; www.fs.fed.us/pnw/corvallis/mdr/mapss/mapss.html) driver for vegetation dynamics] were obtained from the Vegetation/Ecosystem Modeling and Analysis Project (VEMAP) (www.cgd.ucar.edu/vemap). Mammalian species lists for each of the eight national parks were obtained from species lists published by the parks and personal communication with wildlife research personnel within the parks.
Model Details. We first calibrated the park mammalian species lists and the Faunmap data set by determining whether the presence of a species in a park was expected, based on the Faunmap range map for that species. We found no discrepancies between species the parks had identified as present and the ranges depicted by the Faunmap data set. Using an established protocol (11), we next quantified the association between each species' current geographic distribution and VEMAP data on current ecosystem types within each species' entire distribution range by using logistic regression in gis (Geographic Information Systems) (ESRI, Redlands, CA).
Logistic regression requires a priori specification of likelihood for predicting the presence or absence of an entity at a location. We identified a reasonable likelihood value after running a series of calibrations on a set of target species. We used the following steps in our calibrations. We chose a likelihood level, predicted the range distribution for the species, and then compared the predicted range distribution with current mammalian species distributions based on the Faunmap data. We then increased the likelihood value and repeated the calibration steps. We stopped the iteration for a species when 80% or more of the pixels in the predicted and current distribution matched. The likelihood value that produced these results was on average 50%. This value was used in all logistic regressions for all other species used in this assessment. This statistical estimator of each species' habitat associations was then used in conjunction with a new map, depicting ecosystem change under climate warming, to assess the extent to which the geographic range of each species would change. We were forced to discard nine species from our analysis because of an inability to predict reliably their current distribution because of very narrow habitat associations. Eight of the nine species were mice (Rodentia) and shrews (Insectivora) that are habitat specialists: the ninth species was the kit fox (Vulpes velox, Carnivora).
The VEMAP models evaluate distributional change of ecosystems on a single GCM grid cell scale (0.5° Lat × 0.5° Long = 55 km × 35 km), yet many national parks encompass a smaller area. We therefore extended the park boundaries on our gis map by one grid cell in eight compass directions surrounding a park. This expansion effectively permitted us to consider loss in the core area of the park and its greater surrounding park ecosystem (Fig. 1) and reduced the chance that a claim of species loss is simply an artifact of the crudeness of prediction scale. Consequently, our prognosis for species loss from a park will be conservative.
Assessment of Climate Change Effects. We generated the expected distributions under climate change scenarios by applying the regressions of current distribution on current vegetation to VEMAP data onto the distribution of ecosystem types under a doubling of atmospheric CO2. Predicted gains and losses of species from the parks were therefore strictly a function of expected vegetation shifts due to climate change. A species was recorded as potentially present in a park, under the future climate scenario, if acceptable habitat for that species was predicted to occur within park boundaries after a doubling of CO2. In this analysis, we did not consider geographic barriers to dispersal or habitat contiguity.
Maps for current and future mammalian species distributions were overlaid in the gis to identify where major distribution shifts would occur. We then evaluated species losses and gains for each park. We estimated diversity as park species richness the number of mammalian species within a park. For each park, mammalian species that were, according to park records, currently present (current park species) were analyzed separately from all other mammalian species (other species). For each current park species, we queried whether the species' new distribution range would continue to include the park boundaries. If not, this was termed diversity loss. For other species, we queried whether their new distribution would fall within any of the selected park boundaries. If so, this was termed diversity gain. The difference in number of species lost and gained by a park represents its species turnover.
Results and Discussion
Our analysis indicates that if atmospheric CO2 levels double over baseline levels used in current assessments (10), U.S. national parks stand to lose between 0% and 20% of current mammalian species diversity in any one park (the majority of parks stand to lose between 0% and 10%), with an average loss for all parks of 8.3% (Table 1). Big Bend and GSM, the southernmost parks, are projected to suffer the greatest loss of mammalian diversity (20.8% and 16.7%, respectively); at the other extreme, Yellowstone is expected to retain all of the species currently found within its boundaries. These differences between parks arise largely from differences in forecasted changes in vegetation. For example, Big Bend currently contains one ecosystem type, Subtropical Arid Shrubland. This region is expected to be transformed into a C4 grassland (3). Likewise, GSM is currently contained within the Temperate Deciduous Forest ecosystem type, and this region is project to be transformed into Warm Temperate Mixed Forest like that currently found further south (3). Yellowstone, on the other hand, contains a heterogeneous mix of forests and alpine habitat that should not be altered dramatically by climate change (3). The variation of species loss in these parks is a reflection of climate model forecasts that southern ecosystem types will shift northward substantially, and more northerly ecosystem types will remain but become compressed toward the northern boundary of the continental U.S. (10).
Table 1. Current species found in selected U.S. national parks and predicted species losses, gains, and net turnover under a doubling of atmospheric CO2.
| Park | Current species richness* | Species lost | Species gained | Turnover† |
|---|---|---|---|---|
| Acadia | 43 | 3 | 8 | 5 |
| Big Bend | 48 | 10 | 22 | 12 |
| Glacier | 52 | 2 | 45 | 43 |
| GSM | 48 | 8 | 29 | 21 |
| Shenandoah | 33 | 3 | 11 | 8 |
| Yellowstone | 53 | 0 | 49 | 49 |
| Yosemite | 64 | 6 | 25 | 19 |
| Zion | 53 | 1 | 41 | 40 |
Based on park species lists and Faunmap data for mammal species available.
Turnover calculated as species gained minus species lost.
Species losses occurred in all taxonomic orders except Artiodactyla (hoofed mammals). Nearly half of the losses across all parks occurred in rodent species (44%). In addition, 22% of observed losses were Chiropteran species (bats), and 19% of all losses were carnivore species. Examples of sensitive species are the red squirrel (Tamiasciurus hudsonicus), which is expected to be lost from GSM, Shenandoah, and Zion; the southern red-back vole (Clethrionomys gapperi), which will be lost from GSM and Shenandoah; and the northern flying squirrel (Glaucomys sabrinus), which will be lost from GSM. Carnivores most sensitive to climate change are the fisher (Martes pennanti), which will be lost from Acadia and Glacier, and the ringtail (Bassaricus astutus), lost from Yosemite and Big Bend.
Each taxonomic order is expected to lose species in direct proportion to their current relative representation among parks. For example, 42% of the current mammalian species in all parks belong to the order Rodentia, and of the predicted species losses, 44% were rodents. Similarly, Artiodactyla comprise only 6.7% of the total current species found within the parks, and no species from this order are expected to be lost.
Species gains to the parks should outweigh species losses, leading to a positive net turnover (Table 1). We estimated that parks will gain between 11.6% and 92.5% more species relative to current numbers, with an average gain across all parks of 48.1% (Table 2). Species reshuffling is predicted to be dominated by an influx of rodents (40% of all species gained), followed by carnivores, insectivores, and bats (Table 2). This mean turnover compares favorably with estimates for a wider range of taxa in Mexico (12). The projected influx of new species to the parks arises because of range expansion under climate change. That is, most species are expected to remain stable at or near their current geographic locations and to expand their range geographically northward. (A notable exception is the predicted loss of mammalian species from Big Bend and GSM, in response to radical forecasted shifts in vegetation type that necessitate more dramatic range shifts to include southern boundary shifts.) This result is consistent with findings from more regionally focused modeling of climate effects on mammalian species range shifts (13, 14) and is corroborated by empirical observations of climate-induced range shifts in mammals and other taxa (e.g., birds and insects) (15–18). Indeed, in a comprehensive study of boundary shifts for 35 nonmigratory butterfly species, Parmesan et al. (16) concluded that northern boundary extensions were nearly always accompanied by a stable southern boundary, effectively enhancing range size. Similarly, Thomas and Lennon (15) showed that bird species in Great Britain expanded their ranges northward coincident with climate warming, whereas southern margins did not shift systematically northward or southward.
Table 2. Species gains to selected U.S. national parks by taxonomic order under a doubling of atmospheric CO2.
| Park | Total | Chiroptera | Carnivora | Insectivora | Lagomorpha | Rodentia | Artiodactyla | Other |
|---|---|---|---|---|---|---|---|---|
| Acadia | 8 | 1 (12.5) | 3 (37.5) | 2 (25.0) | 0 (0.0) | 1 (12.5) | 0 (0.0) | 1 (12.5) |
| Glacier | 45 | 7 (15.6) | 2 (4.4) | 7 (15.6) | 5 (11.1) | 22 (48.9) | 1 (2.2) | 1 (2.2) |
| Yellowstone | 49 | 6 (12.2) | 4 (8.2) | 10 (20.4) | 4 (8.2) | 23 (26.9) | 1 (2.0) | 1 (2.0) |
| Yosemite | 25 | 2 (8.0) | 3 (12.0) | 5 (20.0) | 1 (4.0) | 13 (52.0) | 1 (4.0) | 0 (0.0) |
| GSM | 29 | 2 (6.9) | 7 (24.1) | 2 (6.9) | 3 (10.3) | 12 (41.4) | 2 (6.9) | 1 (3.4) |
| Shenandoah | 11 | 3 (27.3) | 3 (27.3) | 1 (9.1) | 0 (0.0) | 4 (36.4) | 0 (0.0) | 0 (0.0) |
| Big Bend | 22 | 4 (18.2) | 4 (18.2) | 4 (18.2) | 2 (9.1) | 8 (46.3) | 0 (0.0) | 0 (0.0) |
| Zion | 41 | 3 (7.3) | 5 (12.2) | 7 (17.1) | 3 (7.3) | 19 (40.1) | 2 (4.9) | 2 (4.9) |
Values are numbers and percentages (in parentheses) of species gained.
Our assessment indicates that national parks are not expected to meet their mandate of protecting current mammalian species diversity within park boundaries for several reasons. First, several national parks are expected to face significant losses in current species diversity. Second, all parks should experience a virtual tidal wave of species influxes as a direct consequence of vegetation shifts due to climate change. In the balance, the parks will realize a substantial shift in mammalian species composition of a magnitude unprecedented in recent geological time. This conclusion is based on the assumptions that all species will reshuffle en masse in an orderly manner (19), and that the rate of distribution change is commensurate with geographic shifts in habitat. These assumptions are debatable in general (19), although comparatively rapid (20- to 50-yr) range adjustments are not entirely out of the question for mammals (17, 18).
This assessment represents a first-cut approximation of likely future scenarios and thus provides a conservative prognosis of likely direct effects. In addition, we must consider that, even when significant species losses are not anticipated, there may be repercussions due to indirect effects caused by the reshuffling of mammal communities. As shifting species forge new ecological relationships with each other and with current park species, the character of species interactions and fundamental ecosystem processes stands to become transformed in unforeseen ways (6, 20, 21). For example, an influx of new species may alter existing competitive interactions and influence trophic dynamics with changes in predator–prey interactions. Further, climate warming is likely to result in phenological shifts, including changes in spring breeding dates, flowering, and budburst (6–8), which can further disrupt current species associations. In some cases, it is possible that shifting species assemblages may lead to irreversible state changes, in which the relative abundance of species in different trophic levels can be radically altered (21). The outcome of these new species interactions may be particularly difficult to predict due to the rapid pace of change expected and to the potential for nonlinearities that may emerge, for example, as a consequence of altered trophic interactions.
Our results suggest that the effects of global climate change on wildlife communities may be most noticeable not as a drastic loss of species from their current ranges, but instead as a fundamental change in community structure as species associations shift due to influxes of new species. Obtaining clearer insight into these fine-scale effects rests squarely on developing more detailed models that better account for climate change effects among species and the mosaic of habitat types within a geographic region (6, 11). We hope that this analysis will prompt the next generation of modeling to address the potentially complex indirect effects that may occur as species respond to global climate change.
Nevertheless, our prognosis for the extent and degree of change in park fauna can be viewed as a null hypothesis of distributional expectations (12). More detailed models will likely give finer resolution, but the overall message is likely to be robust. In general, we should observe major changes in mammalian species composition in U.S. national parks due to climate warming. In addition, species interactions could increase the toll of species losses above and beyond what we find in the assessment provided by this study (8, 19).
Acknowledgments
We thank O. Ovadia, E. Palkovacs, D. Skelly, J. Speth, and M. Urban for discussion and comments. This work was supported by a grant from the Edward John Noble Foundation (to O.J.S.) and by a National Science Foundation Graduate Research Fellowship (to C.E.B.).
Abbreviations: VEMAP, Vegetation/Ecosystem Modeling and Analysis Project; GSM, Great Smoky Mountains National Park.
References
- 1.McNeeley, J. & Miller, K., eds. (1994) National Parks Conservation and Development: The Role of Protected Areas in Sustaining Society (Smithsonian Institution Press, Washington, DC).
- 2.Sinclair, A. R. E., Hik, D. S., Schmitz, O. J., Scudder, G. G. E., Turpin, D. H. & Larter, N. C. (1995) Ecol. Appl. 5, 579–587. [Google Scholar]
- 3.VEMAP Members (1997) Global Biogeochem. Cycles 9, 407–437. [Google Scholar]
- 4.Hughes, L. (2000) Trends Ecol. Evol. 15, 56–61. [DOI] [PubMed] [Google Scholar]
- 5.McCarthy, J. P. (2001) Conserv. Biol. 15, 320–331. [Google Scholar]
- 6.Walther, G.-R., Post, E., Convey, P. Menzel, A., Parmesan, C., Beebee, T. J., Fromentin, J.-M., Hoegh-Guldberg, O. & Berlein, F. (2002) Nature 416, 389–395. [DOI] [PubMed] [Google Scholar]
- 7.Parmesan, C. & Yohe, G. (2003) Nature 421, 37–42. [DOI] [PubMed] [Google Scholar]
- 8.Root, T. L., Price, J. T., Hall, K. R., Schneider, S. H., Rosenzweig, C. & Pounds, J. A. (2003) Nature 421, 57–60. [DOI] [PubMed] [Google Scholar]
- 9.Martin, P. H. (1996) Forest Ecol. Manage. 85, 335–341. [Google Scholar]
- 10.National Assessment Synthesis Team (2001) Climate Change Impacts on the United States: The Potential Consequences of Climate Variability and Change (Cambridge Univ. Press, Cambridge, U.K.).
- 11.Johnston, K. M. & Schmitz, O. J. (1997) Global Change Biol. 3, 531–544. [Google Scholar]
- 12.Peterson, A. T., Ortega-Huerta, M. A., Bartley, J., Sanchez-Cordero, V., Soberon, J., Buddemeier, R. H. & Stockwell, D. R. B. (2002) Nature 416, 626–629. [DOI] [PubMed] [Google Scholar]
- 13.Scheel, D., Vincent T. L. S. & Cameron, G. N. (1996) Conserv. Biol 10, 452–464. [Google Scholar]
- 14.Cameron, G. N. & Scheel, D. (2001) J. Mammal. 82, 652–680. [Google Scholar]
- 15.Thomas, C. D. & Lennon, J. J. (1999) Nature 399, 213. [Google Scholar]
- 16.Parmesan, C., Ryrholm, N., Stefanescus, C., Hill, J. K., Thomas, C. D., Descimon, H., Huntley, B., Kaila, L., Kullberg, J., Tammaru, T., et al. (1999) Nature 399, 579–583. [Google Scholar]
- 17.Hersteinsson, P. & MacDonald, D. W. (1992) Oikos 64, 505–515. [Google Scholar]
- 18.Payette, S. (1987) Can. J. Zool. 65, 551–557. [Google Scholar]
- 19.Pimm, S. L. (2001) Nature 411, 531–532. [DOI] [PubMed] [Google Scholar]
- 20.Post, E., Peterson, R. O., Stenseth, N. C. & McLaren, B. E. (1999) Nature 401, 905–907. [Google Scholar]
- 21.Schmitz, O. J., Post, E., Burns, C. E. & Johnston, K. M. (2003) BioScience, in press.