Abstract
Rhagoletis pomonella is a model for incipient sympatric speciation (divergence without geographic isolation) by host-plant shifts. Here, we show that historically derived apple- and ancestral hawthorn-infesting host races of the fly use fruit odor as a key olfactory cue to help distinguish between their respective plants. In flight-tunnel assays and field tests, apple and hawthorn flies preferentially oriented to, and were captured with, chemical blends of their natal fruit volatiles. Because R. pomonella rendezvous on or near the unabscised fruit of their hosts to mate, the behavioral preference for apple vs. hawthorn fruit odor translates directly into premating reproductive isolation between the fly races. We have therefore identified a key and recently evolved (<150 years) mechanism responsible for host choice in R. pomonella bearing directly on sympatric host race formation and speciation.
Speciation in sexual organisms occurs as inherent barriers to gene flow evolve between previously interbreeding populations. To elucidate the origins of species therefore requires understanding how and why new traits arise that reproductively isolate taxa (1). Proponents of sympatric or ecological speciation posit that divergence is often initiated as a result of natural selection differentially adapting populations to alternative habitats (2, 3). Habitat-specific mating is an ecological adaptation central to many models of divergence-with-gene-flow speciation (4, 5). When organisms mate in preferred environments, a system of positive assortative mating is established that helps generate disequilibrium between habitat preference and performance genes. This disequilibrium lessens the “selectionrecombination antagonism” (5, 6), making it potentially possible for divergence to occur without geographic isolation in the face of gene flow (i.e., in sympatry).
The Rhagoletis pomonella sibling species complex is a model for sympatric speciation by host-plant shifts (7). The recently derived apple (Malus pumila)-infesting population of R. pomonella, which originated by a shift from hawthorn (Crataegus spp.) in the mid-1800s, represents an example of host race formation in action, the hypothesized initial stage of sympatric speciation (2, 7). Host-specific mating is a key feature of Rhagoletis biology, as it is for many phytophagous insect specialists (2). Because Rhagoletis flies mate exclusively on or near the unabscised fruit of its host plants (8, 9), differences in host preference translate directly into mate choice and premating reproductive isolation (10). Rhagoletis is a vagile insect; most flies visit multiple trees in their lifetimes searching for food, mates, and fruit oviposition sites (10, 11). The potential therefore exists for substantial mixing between sympatric fly populations. Despite this potential, fly migration has been estimated to be 4–6% per generation per year (Rhagoletis is univoltine) between apple and hawthorn trees based on a mark-recapture experiment conducted at a field site with interspersed host trees (10, 11). Studies on related sibling species in the R. pomonella group have implied that “host fidelity” can potentially cause complete premating isolation between fly taxa (12). Understanding sympatric host race formation and speciation in Rhagoletis, and potentially several other insect specialists, therefore requires elucidating the mechanistic basis for differential host choice. Here we show that host fruit odor plays a key role in this process.
Precisely how R. pomonella distinguishes among potential hosts is not known. However, studies have discerned several cues that apple flies use to recognize apple trees. The major long-range stimulus drawing flies to apple trees appears to be volatile compounds emanating from ripening apple fruit (13). In the field, apple flies oriented upwind toward a point source of butyl hexanoate, a key component of the identified apple volatile blend (Table 1), at a distance of 12 m (14). At shorter distances of <1 m, visual cues become important for finding fruit within the tree canopy (13, 15). Other visual characteristics of trees (e.g., color, shape, and size), although used by flies for distinguishing trees from other objects (16), are not host-specific (13). The literature on host recognition in the R. pomonella apple race therefore suggests that differences in fruit volatiles may be critical for host discrimination.
Table 1. Volatile blends for apple and hawthorn fruit.
| Apple blend | Hawthorn blend |
|---|---|
| Butyl hexanoate (0.37) | Butyl hexanoate (0.01) |
| Pentyl hexanoate (0.05) | 3-Methylbutan-1-ol (1.0) |
| Propyl hexanoate (0.04) | Isoamyl acetate (0.4) |
| Butyl butanoate (0.1) | 4,8-Dimethyl-1,3(E),7-nonatriene (0.02) |
| Hexyl butanoate (0.44) | Ethyl acetate (20.0) |
| Dihydro-β-ionone (0.02) |
The numbers in parentheses are microgram amounts per microliter of the solution applied to the septum.
To determine whether apple and hawthorn flies use fruit odor as an olfactory cue to help distinguish between their host plants, we prepared synthetic blends of apple and hawthorn volatiles that contained the biologically active chemical components of fruit odors (Table 1; refs. 17 and 18). We then used these blends in flight-tunnel assays and field trials to test whether apple- and hawthorn-origin flies preferentially oriented to, or were captured with, their natal fruit volatiles. We report results implying that the historically derived apple fly race has evolved an increased preference for apple fruit volatiles and decreased response to hawthorn volatiles.
Materials and Methods
Insects. Apple and hawthorn flies were collected as larvae from infested fruit in Grant, MI, Fennville, MI, and Urbana, IL, during the 1999–2003 field seasons, and reared to adulthood in the laboratory by using standard protocols (19). Apple and hawthorn populations at these three sites have been the subject of previous ecological and genetic studies and have been shown to differ significantly from one another in allozyme frequencies (20–24). Eclosing adults were kept in cages in an environmental chamber at 23–24°C, 16 h light/8 h dark photoperiod, 60–70% relative humidity, and fed an artificial diet containing water, sugar, vitamins, casein hydrolysate, and a salt mixture (25). Sexually mature, odor-naive adults between 10 and 21 days posteclosion were tested in the flight tunnel. Roughly equal numbers of males and females were tested, and no behavioral difference between the sexes was apparent in the flight tunnel.
Fruit Volatile Blends. Synthetic apple and hawthorn fruit volatile blends were tested in the study (Table 1). The biologically active chemical components of apple and hawthorn fruit volatiles were first identified by using solid-phase microextraction, coupled gas chromatography/electroantennogram detection, mass spectrometry, and a sustained-flight tunnel assay (17, 18). The compositions of the blends were determined through reiterative testing such that equivalent amounts of whole-fruit extracts and the synthetic mixes elicited similar levels of behavioral activity from natal fly races in the flight tunnel (17, 18).
Flight Tunnel. The response of flies to fruit volatiles was measured in a 183-cm-long, 61 × 61-cm-square flight tunnel (see refs. 17 and 18 for details of tunnel and flight conditions). Solutions of the synthetic blends prepared in hexane were applied to acetone-washed, rubber septa (Thomas Scientific, Swedesboro, NJ). A septum was attached to a 7.5-cm-diameter red plastic sphere (Great Lakes IPM, Vestaburg, MI) hung at the upwind end of the tunnel. Individual flies were transferred to a screen cage, which was then placed on a screen stand 1 m downwind of the sphere, and their behaviors were recorded (see Fig. 1 legend for description of fly behaviors). Field experiments have shown that apple flies can orient upwind to a point source of butyl hexanoate, a key volatile of the apple odor blend, at a distance of at least 12 m (14). Fruit volatiles are therefore not just short-range attractants. For all flight-tunnel tests, 200-μg sources of a particular fruit blend were used. For the apple blend, the 200-μg dosage refers to the complete five-component mix (Table 1). For the hawthorn blend, the 200-μg dose reflects the amount of 3-methylbutan-1-ol with the other components added in the proportions shown in Table 1. Blends were prepared 60 min before the tests, with fresh sources and spheres used for each test. Three treatments were tested: (i) a blank red sphere with a control solvent-treated rubber septum, (ii) the apple blend, and (iii) the hawthorn blend.
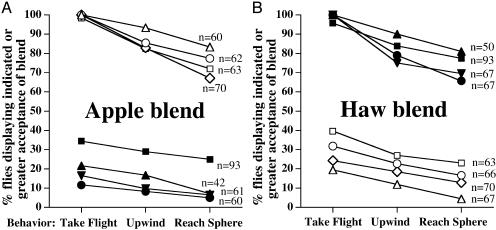
Fig. 1.
Percentages of tested apple- (open symbols) and hawthorn-origin flies (filled symbols) displaying the indicated or greater behavioral acceptance of apple blend (A) and hawthorn (Haw) blend (B) in flight-tunnel assays. Behavioral responses in order of increasing blend acceptance are as follows: walk and groom (fly remaining in release cage), take flight (flight from the release cage), upwind (oriented flight toward sphere), and reach sphere. The percentage of flies displaying walk-and-groom behavior = 100% – % take flight. Populations tested were ▵, Urbana, IL; ▿, Urbana, IL, hawthorn flies reared on apple for two generations; ○, Grant, MI; □, Fennville, MI; and ⋄, Geneva, NY, apple fly colony. P < 1 × 10–7 for every test of behavioral difference between races at a site, as determined by Fisher's exact test. Populations within a race did not differ significantly from each other in their responses to their natal fruit blend. However, significant heterogeneity occurred among apple-fly populations in their responses to hawthorn blend (G test reaching sphere = 11.3, P = 0.158, 3 df) and among hawthorn fly populations to apple blend (G test = 15.8, P = 0.0006, 2 df).
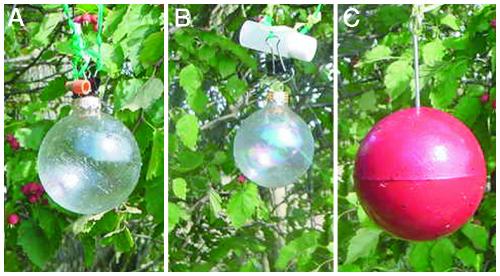
Field Trials. Field trapping studies were conducted in mixed-variety apple orchards and hawthorn copses from August 27 to September 9, 2002, at the Experiment Station in Geneva, NY, and from July 25 to September 5, 2002, at the Trevor Nichols Research Complex near Fennville, MI. Red sphere traps (7.5-cm diameter) coated with “Tanglefoot” stickum were used in New York, whereas clear glass spheres (5.5-cm diameter) were used at the Michigan site to remove any visual cue provided by the red sphere. (Fig. 2 shows the spheres used in the study.) Three-way choice experiments were performed to assess the relative preferences of the host races for fruit odors. For the three-way tests, rubber septa lures containing 2 mg of apple, hawthorn, or no blend were separately attached to the tops of three spheres triangulated 2 m apart in host trees. Three replicate tests were conducted at each site in a trial period, with a trial period lasting from 1 to 2 days. Traps were checked after each trial period, with captured flies counted and removed, lures replaced, and traps rotated to new positions. Statistical analyses were performed by using the total number of flies captured across the three replicates during trial periods. Paired field trials of only the apple blend vs. blank controls on clear spheres were also performed at the Fennville, MI, site to assess host race attraction to apple odor in the absence of the visual cue provided by the red sphere. For the paired experiments, the apple blend was released from scintillation vials prepared by Great Lakes IPM. Release rate of odor from these vials was estimated at 1 mg/h at 25°C. Baited, clear spheres were hung 1 m from blank clear spheres fitted with empty vials. Six pairs of replicate traps were monitored and rotated every 5 days for the paired trials. The same design was used to test the apple blend in flowering dogwood (Cornus florida) stands in Cassopolis, MI, and Granger, IN, from September 16 to October 13, 2002.
Fig. 2.
Tanglefoot-coated spheres used for field trials. (A) Clear sphere with septum used to release volatiles for three-way choice tests at Fennville, MI. (B) Clear sphere with scintillation vial used to release volatiles for paired apple-blend vs. blank tests. (C) Red sphere used in New York field trials and flight tunnel. Background is a hawthorn tree with red fruits visible.
Results and Discussion
Flight Tunnel. In control flight-tunnel experiments, no fly of either host race flew upwind toward a “blank” red sphere fitted with an odorless septum. The sphere and septum used as a release point for the blends in the tunnel therefore held no intrinsic attractive value from the 1-m distance at which flies were released.
Significant differences were observed, however, in the behavioral responses of the host races to red spheres with apple vs. hawthorn volatiles. Virtually every apple-origin fly tested took flight when the septum attached to the sphere contained the apple blend. A majority of these apple flies (<70%) displayed upwind anemotactic flight, tracking the apple-odor plume in the tunnel to reach the source sphere (Fig. 1 A). The finding of anemotactic flight, not previously reported for Rhagoletis, is important because it implies that these flies have the capacity to locate an olfactory source from a considerable distance in the field. Hawthorn-origin flies responded similarly when the sphere contained the hawthorn blend (Fig. 1B). However, both fly races displayed a significantly reduced response to their nonnatal blend. Less than 25% of apple flies flew upwind and reached the sphere when hawthorn volatiles were present (Fig. 1B), and fewer hawthorn flies reached apple-blend spheres (Fig. 1 A). The results were similar for three pairs of apple and hawthorn fly populations tested from Grant, MI, Fennville, MI, and Urbana, IL, and for a laboratory colony of Geneva, NY, apple flies established from the wild in the 1970s (Fig. 1). Thus, the host races showed a consistent pattern of preference for their natal vs. nonnatal blend across their geographic range of overlap. Moreover, Urbana, IL, hawthorn flies reared for two generations in the laboratory on apple displayed the same behavioral responses as hawthorn flies reared directly from field-collected hawthorns (Fig. 1). This finding discounts an effect of the larval-host fruit environment on adult fly behavior. Genetic crosses between apple and hawthorn flies are expected to allow mapping of quantitative trait loci for host odor preference.
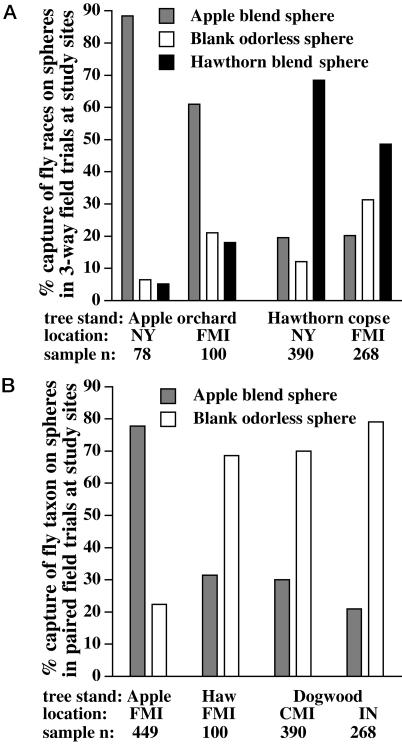
Three-Way Choice Study. Field trials indicated that the preferences displayed by the host races in the flight tunnel were relevant in nature. In three-way choice experiments conducted in unsprayed apple orchards near Geneva, NY, and Fennville, MI, resident flies were captured significantly more often on sticky spheres (red in New York, clear in Michigan) baited with the apple-blend than on hawthorn-blend or blank spheres (Figs. 2 and 3A; χ2r Friedman's test for the New York apple orchard was 21.1; P < 0.0001 for significantly higher rank order capture on apple-blend spheres across 12 replicate block periods; χ2r for the Michigan apple orchard was 6.0; P = 0.05, three replicate periods). The pattern was reversed at hawthorn tree stands in New York and Michigan <1 km away from the apple orchards (Fig. 3A). Here, significantly more flies were trapped on the hawthorn blend than the other spheres (χ2r New York hawthorn stand was 12.7, P < 0.001, 12 replicate periods; χ2r for the Michigan hawthorn stand was 6.0, P = 0.05, 3 replicate periods).
Fig. 3.
Total percentages of resident Rhagoletis flies captured across replicate trapping periods on baited spheres (red in New York, clear in Michigan and Indiana) in apple orchards, hawthorn copses, and dogwood-tree stands in Geneva, NY, Fennville, MI (FMI), Cassopolis, MI (CMI), and Granger, IN. (A) Results for three-way choice study of apple blend, hawthorn blend, and blank spheres. P < 1 × 10–12 for all pairwise comparisons of difference in fly capture on sphere types between apple orchard and hawthorn tree copses, as determined by G contingency tests. (B) Results for paired field study of apple blend vs. blank, clear spheres in apple, hawthorn, and dogwood tree stands. P < 1 × 10–15 for all comparisons of difference in capture on sphere types between apple vs. hawthorn or dogwood stands, as determined by two-tailed Fisher's exact test. Sample n = total number of flies trapped on all spheres in a given tree stand.
Paired Field Trials. The flight-tunnel and three-way choice experiments imply that the historically derived apple race has evolved an increased preference for apple volatiles. To further test this hypothesis, we performed paired field trails of just the apple blend vs. blank clear spheres at the Fennville, MI, site (Fig. 2B), and a study of R. pomonella's sister species, the undescribed flowering dogwood fly (26). In the Fennville apple orchard, significantly more resident flies were captured on the apple blend than blank spheres (Fig. 3B; Z = 2.93, P = 0.003, two-tailed Wilcoxon sign-rank test for greater capture on apple blend spheres across 11 replicate periods). In the hawthorn tree copse, in contrast, significantly more flies were captured on blank spheres than on apple-blend spheres (Fig. 3B; Z = 2.52, P = 0.012, eight replicate periods). The results in flowering dogwood stands were similar to those for hawthorn trees (Fig. 3B). At two stands of C. florida trees near Granger, IN, and Cassopolis, MI, a total of 58 resident flies were captured on apple-blend vs. 175 on blank spheres (Z = 2.52, P = 0.012, eight replicate periods in Indiana; Z = 2.02, P = 0.043, five replicate periods in Michigan). The reduced capture of both the ancestral hawthorn race and immediate outgroup dogwood fly on apple-blend vs. blank clear spheres supports the hypothesis that the increased preference of apple flies for apple odor is a derived characteristic of the population. The results also suggest that hawthorn and dogwood flies may avoid the odor of apples.
Conclusion. Our results imply that the apple race of R. pomonella has evolved an increased preference for a specific blend of volatiles from apple fruit, and decreased response to hawthorn volatiles, during the course of its ≈150 years of existence. Because mate choice in R. pomonella is directly tied to host choice, the difference in host odor preference results in premating reproductive isolation between apple and hawthorn flies. We have therefore identified a key host-related adaptation underlying host race formation and incipient sympatric speciation in R. pomonella.
In conclusion, investigations of the apple maggot fly are adding to a growing list of systems demonstrating a role for ecological adaptation in incipient population divergence and speciation (3, 27–29). What makes the R. pomonella story compelling is that the known history and geography of race formation allows us to directly connect host adaptation (e.g., fruit-odor preference) and reproductive isolation in real-time ecological experiments in nature.
Acknowledgments
We thank K. Catropia, K. Filchak, R. Harrison, C. Musto, R. Oakleaf, K. Pelz, K. Poole, H. Reissig, J. Roethele, C. Smith, L. Stelinski, U. Stolz, B. Westrate, J. Wise, the Niles, MI, U.S. Department of Agriculture facility, the Trevor Nichols Research Complex, and the New York State Agricultural Experimental Station at Geneva Fly Rearing Center. This work was supported by grants from the National Science Foundation Integrated Research Challenges (to all authors) and the U.S. Department of Agriculture National Research Initiative (to J.L.F. and S.H.B.) and by the state of Indiana 21st Century Fund (to J.L.F.).
References
- 1.Coyne, J. A. (1992) Nature 355, 511–515. [DOI] [PubMed] [Google Scholar]
- 2.Bush, G. L. (1969) Am. Nat. 103, 669–672. [Google Scholar]
- 3.Schluter, D. (2001) Trends Ecol. Evol. 16, 372–380. [DOI] [PubMed] [Google Scholar]
- 4.Johnson, P. A. & Gullberg, U. (1998) in Endless Forms: Species and Speciation, eds. Howard, D. J. & Berlocher, S. H. (Oxford Univ. Press, New York), pp. 79–89.
- 5.Fry, J. D. (2003) Evolution (Lawrence, Kans.) 57, 1735–1746. [DOI] [PubMed] [Google Scholar]
- 6.Felsenstein, J. (1981) Evolution (Lawrence, Kans.) 35, 124–138. [DOI] [PubMed] [Google Scholar]
- 7.Bush, G. L. (1966) Bull. Mus. Comp. Zool. 134, 431–562. [Google Scholar]
- 8.Prokopy, R. J., Bennett, E. W. & Bush, G. L. (1971) Can. Entomol. 103, 1405–1409. [Google Scholar]
- 9.Prokopy, R. J., Bennett, E. W. & Bush, G. L. (1972) Can. Entomol. 104, 97–104. [Google Scholar]
- 10.Feder, J. L., Opp, S., Wlazlo, B. Reynolds, K., Go, W. & Spisak, S. (1994) Proc. Natl. Acad. Sci. USA 91, 7990–7994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feder, J. L., Berlocher, S. H. & Opp, S. B. (1998) in Genetic Structure in Natural Insect Populations: Effects of Host Plants and Life History, eds. Mopper, S. & Strauss, S. (Chapman & Hall, New York), pp. 408–441.
- 12.Feder, J. L. & Bush, G. L. (1989) Evolution (Lawrence, Kans.) 43, 1813–1819. [DOI] [PubMed] [Google Scholar]
- 13.Prokopy, R. J. & Roitberg, B. D. (1984) Am. Sci. 72, 41–49. [Google Scholar]
- 14.Green, T. A. & Prokopy, R. J., J. Chem. Ecol., in press.
- 15.Aluja, M., Prokopy, R. J., Elkinton, J. S. & Laurence, F. (1989) Environ. Entomol. 18, 1–7. [Google Scholar]
- 16.Prokopy, R. J., Moericke, V. & Bush, G. L. (1973) Environ. Entomol. 2, 743–749. [Google Scholar]
- 17.Zhang, A., Linn, C., Jr., Wright, S., Prokopy, R., Reissig, W. H. & Roelofs, W. L. (1999) J. Chem. Ecol. 25, 1221–1232. [Google Scholar]
- 18.Nojima, S., Linn, C., Jr., Morris, B., Zhang, A. & Roelofs, W. L. (2003) J. Chem. Ecol. 29, 319–334. [DOI] [PubMed] [Google Scholar]
- 19.Feder, J. L., Chilcote, C. A. & Bush, G. L. (1989) J. Hered. 80, 277–283. [Google Scholar]
- 20.Feder, J. L., Chilcote, C. A. & Bush, G. L. (1988) Nature 336, 61–64. [Google Scholar]
- 21.McPheron, B. A., Smith, D. C. & Berlocher, S. H. (1988) Nature 336, 64–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Feder, J. L. & Bush, G. L. (1989) Heredity 63, 245–266. [Google Scholar]
- 23.Feder, J. L., Chilcote, C. A. & Bush, G. L. (1990) Evolution (Lawrence, Kans.) 44, 570–594. [DOI] [PubMed] [Google Scholar]
- 24.Feder, J. L. & Bush, G. L. (1990) Evolution (Lawrence, Kans.) 44, 595–608. [DOI] [PubMed] [Google Scholar]
- 25.Neilson, W. T. A. & McAllen, J. W. (1965) J. Econ. Entomol. 58, 542–543. [Google Scholar]
- 26.Berlocher, S. H. (2000) Evolution (Lawrence, Kans.) 54, 543–557. [DOI] [PubMed] [Google Scholar]
- 27.Orr, M. R. & Smith, T. B. (1998) Trends Ecol. Evol. 13, 502–506. [DOI] [PubMed] [Google Scholar]
- 28.Via, S. (2001) Trends Ecol. Evol. 16, 381–390. [DOI] [PubMed] [Google Scholar]
- 29.Berlocher, S. H. & Feder, J. L. (2002) Annu. Rev. Entomol. 47, 773–815. [DOI] [PubMed] [Google Scholar]