Abstract
Cysteinyl leukotrienes (cysLTs) mediate vascular leakage and bronchoconstriction through the smooth muscle-associated CysLT type 1 receptor (CysLT1R), one of at least two loosely homologous cysLT-binding G protein-coupled receptors. We previously reported that CysLT1R is expressed by cultured human mast cells (hMCs), and that priming these cells with IL-4 enhances their sensitivity to calcium flux and cytokine generation in response to cys-LTs and the nucleotide ligand, uridine diphosphate (UDP), without increasing their surface expression of CysLT1R. We now report that hMCs express the type 2 receptor for cysLTs (CysLT2R) as well, and that the amount of surface CysLT2R protein increases in response to priming with IL-4. The selective function of CysLT2R was evident based on uninhibited IL-8 secretion by IL-4-primed hMCs stimulated with cys-LTs or UDP in the presence of the selective CysLT1R antagonist MK571. MK571 did inhibit IL-5 generation, calcium flux, and phosphorylation of extracellular signal-regulated kinase. IL-8 secretion was inhibited by pertussis toxin and a selective p38 kinase inhibitor, SB203580. The CysLT2 response may permit the cys-LTs and nucleotides generated in infection and tissue injury to elicit IL-8 generation by hMCs, potentially leading to neutrophilic infiltration, a characteristic of aerosol challenge-induced late-phase responses and of sudden death associated with asthma.
The cysteinyl leukotrienes (cysLTs) are a class of lipid inflammatory mediators formed from arachidonic acid by activated mast cells (MCs), eosinophils, basophils, and alveolar macrophages. In addition to potent effects on vascular smooth muscle (1–3), cysLTs are powerful bronchoconstrictors in vivo (4, 5), accounting for ≈50% of the decrease in the forced expiratory volume at 1 sec (FEV1) that occurs during allergen-induced early phase airflow obstruction in individuals with allergic asthma (6). The effects of the cysLTs are mediated through two known receptors: the CysLT type 1 receptor (CysLT1R) which accounts for the bronchoconstricting effects of the cysLTs (6–8), and the CysLT type 2 receptor (CysLT2R). The cysLTs also act on inflammatory cells, recruiting both eosinophils and neutrophils when instilled into the airways of human subjects (9). Furthermore, the selective antagonism of CysLT1R decreases both sputum and peripheral blood eosinophil counts (10), a finding that may relate to the ability of cysLTs to induce cytokine production by resident lung cells (11). Although CysLT2R is coexpressed with CysLT1R on several immune cells (12–15), no specific functions have been reported for CysLT2R.
CysLT1R and CysLT2R are only loosely homologous (38% amino acid identity) (12, 13, 16, 17) and each also shares a similar degree of identity (24–32%) with the purinergic (P2Y) class of G protein-coupled receptors (GPCRs) that mediate cellular responses to extracellular nucleotides (18). CysLT1R and CysLT2R have different ligand affinities when expressed heterologously. CysLT1R binds leukotriene D4 (LTD4) with a 10-fold higher affinity than it binds leukotriene C4 (LTC4) (EC50 ≈ 10–9 M vs. 10–8 M, respectively), whereas CysLT2R binds LTC4 and LTD4 equally (EC50 ≈ 10–8 M) (12, 13).
We previously reported (19) that cord blood-derived human MCs (hMCs) express CysLT1R protein and mRNA and respond to cysLTs with a strong and sustained calcium flux that is completely blocked by pretreatment of the cells with MK571, a CysLT1R-selective competitive antagonist. CysLT-mediated calcium flux is resistant to treatment with pertussis toxin (PTX), consistent with the coupling of the CysLT1R to PTX-resistant Gq proteins in hMCs, as reported for Xenopus oocytes transfected with human CysLT1R (12). When primed with IL-4, the sensitivity of hMCs to LTC4 is markedly enhanced (and to a greater extent than their sensitivity to LTD4). Unexpectedly, IL-4 priming also enhances the calcium response of hMCs to uridine diphosphate (UDP), and UDP desensitizes the hMCs to LTC4-induced calcium flux, but not to LTD4. Moreover, IL-4 primed hMCs (but not their unprimed replicates) secrete IL-5, tumor necrosis factor (TNF)-α, and macrophage inflammatory protein (MIP)-1β in response to stimulation with LTC4, LTD4, or UDP, without undergoing exocytosis (20). Production of all three of these cytokines by IL-4-primed hMCs in response to cysLTs and UDP is inhibited by the calcineurin inhibitor FK506, by the selective inhibitor of extracellular regulated kinase (ERK) UO126, and by MK571, indicating ligand recognition by CysLT1R or a CysLT1R-like receptor.
Because the marked IL-4-induced enhancement of the calcium flux induced by LTC4 and UDP did not involve changes in surface levels of CysLT1R, and because the partial CysLT2R agonist BAY-u9773 induced a small calcium flux in hMCs, we hypothesized the presence of a second functional CysLTR on hMCs. We now report that cord blood-derived hMCs constitutively express CysLT2R and that IL-4 consistently up-regulates the surface expression of CysLT2R on a fraction of hMCs. In these hMCs, MK571 completely blocked the cysLT-initiated calcium response and generation of IL-5 and the LTC4-induced phosphorylation of ERK. However, MK571 did not interfere with sustained LTC4-induced p38 mitogen-activated protein kinase (MAPK) phosphorylation and left cysLT-and UDP-dependent IL-8 generation unaffected. The partial CysLT2R agonist BAY-u9773 also induced IL-8 generation, indicating that IL-8 is a “signature” cytokine of CysLT2R-mediated hMC activation. Moreover, IL-8 generation was inhibited by PTX, implying that CysLT2R uses Gi/Go proteins for calcium-independent signaling in hMCs. Thus, CysLT1R and CysLT2R have nonredundant functions on hMCs, coupling to distinct intracellular signaling pathways. Moreover, coexpression of both CysLTRs may facilitate cooperative recognition of low concentrations of ligand and enhanced signaling for cytokine generation in circumstances of inflammation or tissue injury.
Materials and Methods
Calcium Mobilization and Cytokine Generation. hMCs were derived from cord blood mononuclear cells by culture as described (21). Cells were harvested when >95% stained positively with toluidine blue, and were further cultured with either stem cell factor (SCF, 100 ng/ml, R & D Systems) alone or with SCF and IL-4 (10 ng/ml, R & D Systems) for 5 days at 37°C and 5% CO2. Changes in the cytostolic free Ca2+ concentration elicited by LTC4 and LTD4 (both from Cayman Chemical, Ann Arbor, MI) were measured with fura 2-loaded hMCs (≈0.5 × 106 cells per sample) as described (19).
For cytokine production, IL-4-primed hMCs were stimulated with LTC4, LTD4, UDP (Sigma), or BAY-u9773 (Biomol, Plymouth Meeting, PA) in triplicate samples of 1 × 105 hMCs in the wells of 96-well flat bottom plates as described (20). SCF was included in all stimulation conditions to ensure optimal survival over the 6-h stimulation period. Supernatants were stored at –20°C until analysis by commercial ELISA (Endogen, Woburn, MA). Broad-based cytokine analysis (Perbio, Endogen) was performed commercially. The cytokine quantities in the samples stimulated with media without agonists were subtracted from the corresponding quantities in the samples stimulated with agonists to obtain the net quantity produced for each cytokine. In some experiments, hMCs were incubated overnight in the presence of PTX (100 ng/ml, Sigma), or for 5 min with the selective p38 antagonist SB203580 (10 μM, Promega) before activation.
MAPK Measurements. MAPK phosphorylation was assessed as described (20) in response to LTC4 and BAY-u9773, both with and without pretreatment of the cells for 10 min with MK571 (1 μM, Cayman Chemical). SDS/PAGE immunoblots were probed with Abs specific for total ERK, p38, and c-Jun-NH2-terminal kinase (JNK) MAPKs (Cell Signaling Technologies) and their respective phosphorylated isoforms (Promega) as described (20).
Measurement of CysLT2R Protein and mRNA Expression. Cytofluorographic analysis was performed on saponin-permeabilized and nonpermeabilized hMCs. Samples of 3 × 105 cells were stained with 2 μg of polyclonal Abs raised against N-terminal or C-terminal peptides of the human CysLT2R, or against a C-terminal peptide from CysLT1R, or with an equivalent amount of a nonspecific rabbit IgG (all from Cayman Chemical) and analyzed as described (21). Immunohistochemistry and chloroacetate esterase staining was performed on surgically excised nasal polyps as reported (22). Tissue sections were stained with 1:50 dilutions of the anti-CysLT2 C-terminal peptide Ab, with a mAb to human α and β tryptases (Calbiochem), or with equivalent dilutions of isotype-matched controls. Staining of CysLT2R was localized to hMCs by using overlaying of images from adjacent sections. For assessment of total cellular CysLT2R content, whole hMC lysates were prepared and resolved on precast SDS/14% polyacrylamide gels (NOVEX, San Diego) (23). Immunoblots were probed with 1:500 dilutions of anti-CysLT2R C-terminal Ab, followed by a horseradish peroxidase-conjugated goat anti-rabbit secondary Ab. Bands were detected by autoradiography using enhanced chemiluminescence (Amersham Pharmacia). The expression of CysLT1R and CysLT2R mRNA was assessed by real-time PCR (TaqMan, Applied Biosystems) as described (24).
Results and Discussion
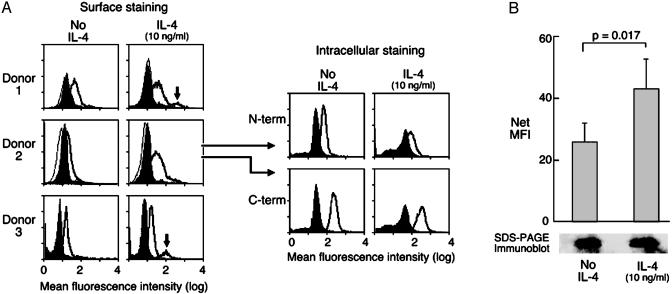
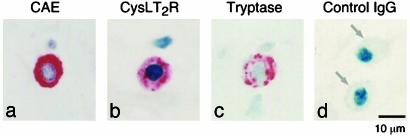
We first performed a cytofluorographic analysis on cultured hMCs by using two polyclonal anti-peptide Abs, directed at CysLT2-specific N- and C-terminal sequences, respectively. Both permeabilized and nonpermeabilized hMCs were studied to visualize both intracellular and surface-associated CysLT2R protein (Fig. 1A). The specificity of the staining was supported by the fact that the anti-C-terminal Ab raised against an epitope predicted to reside in the cytosolic tail of the receptor stained only the permeabilized hMCs. Resident hMCs in two surgically excised nasal polyps showed immunoreactivity for CysLT2R with both Abs (as shown for the C-terminal Ab in one donor, Fig. 2). Both the pattern of immunohistochemical staining in the polyp hMCs and the enhanced cytofluorographic staining of permeabilized cultured hMCs indicated that a significant portion of CysLT2R was located intracellularly in hMCs, even in a donor with very low surface expression at baseline (donor 2, Fig. 1 A). Thus, hMCs express both known CysLTRs, as has been reported for eosinophils and macrophages (13, 14).
Fig. 1.
Expression of CysLT2R by hMCs. (A) Cytofluorographic surface staining for CysLT2R (bold tracings) by using a rabbit-anti-human IgG directed at an N-terminal epitope. hMCs were treated for 5 days with SCF only (100 ng/ml) or SCF plus IL-4 (10 ng/ml). Histograms are overlayed on tracings elicited by an IgG control (shaded) and by secondary Ab alone (light tracings). Results from three separate donors (of five tested) are displayed. Intracellular staining on saponin-permeabilized hMCs with the same anti-N-terminal IgG (N-term), and with an anti-C-terminal IgG (C-term). Results depicted for intracellular staining are from the cells of donor 2 and are representative of results from four separate donors. (B) Changes in cytofluorographic surface expression of CysLT2R induced by IL-4 priming, as determined by net MFI. Results are the mean ± SEM of data from five separate experiments. SDS/PAGE immunoblot (Bottom) from one of these experiments is displayed, depicting the CysLT2R signals for nonprimed and IL-4-primed samples. Results are representative of three experiments.
Fig. 2.
Immunoreactivity for CysLTRs on nasal polyp hMCs. Sections through surgically excised polyps were stained for chloroacetate esterase (CAE) (a) and for CysLT2R with the anti-C-terminal Ab (b). (c) The cells were identified as hMCs in serial sections based on CAE staining and tryptase immunostaining of adjacent sections. (d) Negative control, performed on hMCs in a different section, is indicated. Results are representative of experiments performed with polyps from two different patients.
Because IL-4 priming increases the sensitivity of hMCs to CysLTR-mediated signaling without altering levels of CysLT1R expression (20), we determined whether this priming event changed membrane expression of CysLT2R protein. Cytofluorographic analysis of hMCs from 5 consecutive donors revealed a modest but significant enhancement of CysLT2R membrane staining in hMCs treated for 5 d with recombinant human IL-4 (10 ng/ml) in the presence of SCF, compared with replicates maintained in cytoprotective SCF alone (net MFI 43 ± 10 vs. 26 ± 6 arbitrary units, P = 0.017, mean ± SEM, n = 5, Fig. 1B). In four of these five donors, the increase over the baseline surface expression was caused by the induction of high levels in a subgroup of hMCs by IL-4 (≈10–15% of the total population). In the other donor (donor 2), surface expression of CysLT2R was low in the absence of priming, and IL-4 induced an upward shift in the cytofluorographic peak for CysLT2R in the entire population. IL-4 priming did not substantially alter levels of CysLT2R staining in permeabilized hMCs using either Ab (as shown for donor 2 in Fig. 1 A), did not change the substantial intensity of baseline immunodetectable CysLT2R protein on SDS/PAGE immunoblot (Fig. 1B), and did not change steady-state levels of CysLT2R mRNA as measured by real-time PCR (not shown). It is thus likely that the change in surface CysLT2R largely reflects a redistribution of CysLT2R to the plasma membrane. In agreement with results reported previously (20), CysLT1R protein and transcript were also expressed by hMCs, and neither was altered by IL-4 priming (n = 3, data not shown).
To determine whether CysLT2R has distinct functions, we studied ligand-initiated responses in the presence and absence of the CysLT1R-selective antagonist, MK571. As in our previous studies (20), IL-4 priming substantially enhanced cysLT-mediated calcium fluxes of hMCs (data not shown). Nevertheless, MK571 at 1 μM completely blocked the ability of IL-4-primed hMCs to flux calcium in response to LTC4 (n = 3, as shown for one experiment in Fig. 3A) at doses as high as 1 μM, despite their considerable expression of CysLT2R. Although it is possible that the capacity of IL-4 to modestly up-regulate CysLT2R membrane expression on hMCs is unrelated to its enhancement of cysLT-mediated calcium flux, it is also possible that CysLT2R cooperates with CysLT1R in the recognition of cysLTs (LTC4 in particular), possibly by formation of an MK571-sensitive CysLT1R/CysLT2R heterodimer similar to those described in other GPCR systems (25).
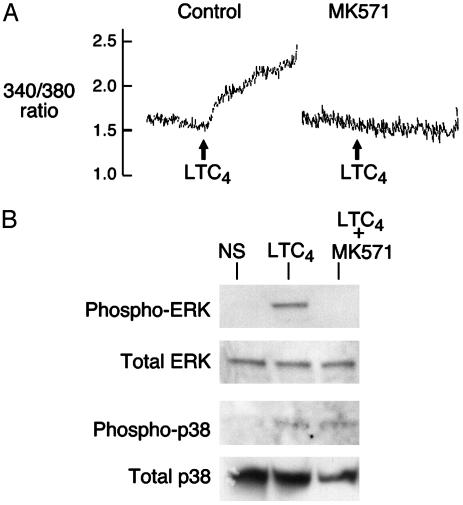
Fig. 3.
Calcium flux and MAPK phosphorylation in response to CysLTR ligation in hMCs. (A) Complete blockade of LTC4-induced calcium flux (at 10–7 M) in IL-4-primed hMCs by MK571 (10–6 M). hMCs were primed for 5 days with recombinant IL-4 in the presence of SCF, loaded with fura 2-AM dye, and stimulated with LTC4. Results were identical in a two additional experiments with cells from different donors and replicated earlier data (19). (B) MAPK phosphorylation in IL-4-primed hMCs stimulated for 5 min (ERK blots) or 30 min (p38 blots) with LTC4 (10–7 M) with or without pretreatment with MK571 (10–6 M). Results are from one experiment representative of two performed.
The absence of a calcium flux in the MK571-treated, LTC4-stimulated hMCs led us to explore whether CysLT2R, functioning independently in hMCs, might use signals not dependent on phospholipase (PL)C and calcium flux. As reported previously (20), stimulation of primed hMCs for 5 min with LTC4 (10–7 M) resulted in robust phosphorylation of ERK. Pretreatment with MK571 (10–6 M) completely inhibited ERK phosphorylation (n = 3, as shown for 1 donor, Fig. 3B). Thus as with calcium flux, cysLT-mediated ERK phosphorylation in hMCs requires binding to CysLT1R. p38 MAPK was phosphorylated even in unstimulated hMCs and did not differ from the signal obtained from 5 min of LTC4 stimulation. However, at 30 min, when the signal in the unstimulated samples was virtually undetectable, LTC4 induced incremental, MK571-resistant phosphorylation of p38 (n = 2, as shown for one donor, Fig. 3B), suggesting that the two known receptors for cysLTs couple to different MAPK signaling cascades in hMCs.
We next sought differences in secretory functions between the two CysLTRs. In our previous studies, MK571 significantly blocked cysLT-induced generation of IL-5, TNFα, and MIP-1β by 80% or more, indicating the requirement for CysLT1R (or CysLT1R-like) receptors for this response (20), while, in retrospect, revealing a potential, small CysLT2R-dependent residual signal. Therefore, we assayed supernatants from IL-4-primed hMCs that had been stimulated for 6 h with cysLTs (10–7 M each) in the presence or absence of MK571 at a 10-fold molar excess for multiple cytokine and chemokine products, by using a broad-based ELISA measurement of multiple cytokine and chemokine products in the same samples (Perbio System, Endogen). The secretion of most of the cytokines and chemokines by IL-4-primed, cysLT-stimulated hMCs was completely or markedly inhibited by MK571 pretreatment. Noteworthy exceptions included IL-16 and the chemokines monocyte chemoattractant protein-1 and IL-8.
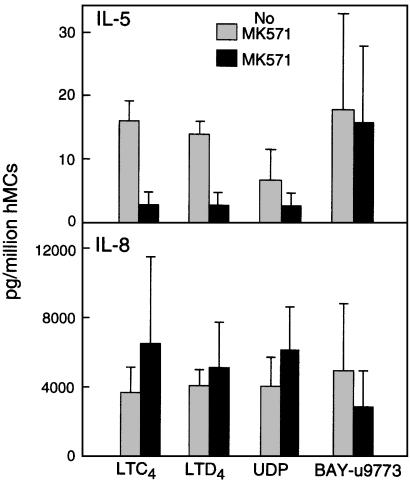
We focused our further analyses on the CysLT2R-dependent generation of IL-8 because of the robust nature of this signal, and contrasted it with the secretion of IL-5 to serve as a control for the efficacy of the CysLT1R blockade. The blockade of IL-5 secretion by IL-4-primed hMCs stimulated with LTC4, LTD4, and UDP by a 10-fold molar excess of MK571 contrasted sharply with the complete lack of blockade for IL-8 secretion (Fig. 4). BAY-u9773 at 5 × 10–6 M elicited secretion of IL-8 at levels comparable to those elicited by stimulation with cysLTs or UDP, and also induced IL-5 generation. Pretreatment with PTX or with the p38 inhibitor SB203580 virtually abolished ligand-induced IL-5 and IL-8 generation, both with and without MK571 pretreatment (n = 3, data not shown). Thus, activation of hMCs through the CysLT2R accounts for the entirety of cys-LT-dependent IL-8 generation, thereby establishing the unique effector function of this receptor under conditions where CysLT1R is blocked. The fact that IL-5 generation occurs as a result of stimulation with BAY-u9773, but not cys-LTs under conditions where CysLT1R is blocked, suggests unique agonistic properties of this compound, possibly by stimulating a CysLT1/CysLT2 heterodimer at a site inaccessible to interference from MK571. Moreover, the complete inhibition of CysLT2-dependent IL-8 secretion by PTX implies that CysLT2R uses Gi/o family proteins to induce secretion in hMCs. This contrasts with the reported utilization of Gq proteins by the cloned CysLT2R transfected into Xenopus oocytes (13), and emphasizes that G protein coupling of a single receptor may differ between cell types. G proteins of the Gi/o class mediate the function of the P2Y12 receptor, a homologue of CysLT1R and CysLT2R, without PLC activation and calcium flux to adenosine diphosphate (26). Under conditions without receptor blockade, the composite effects of cysLT-induced hMC activation thus reflect contributions of both CysLT1R (Gq activation, calcium flux, ERK phosphorylation) and CysLT2R (Gαi/o activation, p38 phosphorylation). When CysLT1R is blocked, CysLT2R is still functional and induces a distinct profile of biochemical events and secreted products, even though cysLTs cannot induce calcium flux in hMCs under such circumstances. The fact that IL-5 generation is blocked by MK571 and by inhibitors of downstream signaling for either CysLT1R or CysLT2R suggests synergy and cooperation of the two receptors after ligand recognition.
Fig. 4.
Effects of MK571 on the production of IL-5 (Upper) and IL-8 (Lower) by hMCs stimulated for 6 h with LTC4, LTD4, or UDP (10–7 M each) or BAY-u9773 (5 × 10–6 M). The results for LTC4, LTD4, and UDP-induced IL-8 generation are the mean ± SEM for five experiments, including the three used to measure IL-5. Data with BAY-u9773 are the mean ± SEM from three experiments. Both IL-5 and IL-8 generation were completely inhibited by PTX (n = 3 and data not shown).
Our study identifies a distinct function for the CysLT2R. CysLT2R alone accounts for a small MK571-resistant residual IL-5 generation in response to cysLTs and UDP, and for the entirety of IL-8 production in response to these ligands (Fig. 4). IL-8 is a major chemoattractant that may relate to the recruitment of neutrophils to the airways of allergic individuals in response to experimental allergen challenge, and to the neutrophil dominance of bronchial pathology in patients who die suddenly of status asthmaticus (27). During inflammatory responses, cys-LTs may be abundant because of their generation by MCs, macrophages, and eosinophils, whereas extracellular nucleotides, including UDP, would be prominent products of cellular damage and microbial invasion. Each class of ligand could mediate autocrine and paracrine extracellular signaling in hematopoietic cells expressing both CysLTRs. Moreover, the incomplete therapeutic effects of selective CysLT1R antagonists could reflect their ability to interfere with some, but not all, effector functions of the CysLTR ligands on hMCs and other resident tissue cells that express both receptors.
Acknowledgments
This work was supported by National Institutes of Health Grants AI-48802, AI-52353, AI-31599, and HL-36110, and by grants from the Charles Dana Foundation, the Vinik Family Fund for Research in Allergic Diseases, and the Hyde and Watson Foundation.
Abbreviations: CysLTs, cysteinyl leukotrienes; CysLT2R, type 2 receptor for cysLTs; CysLT1R, type 1 receptor for cysLTs; LTD4, leukotriene D4; LTC4, leukotriene C4; MCs, mast cells; hMCs, human MCs.
References
- 1.Soter, N. A., Lewis, R. A., Corey, E. J. & Austen, K. F. (1983) J. Invest. Dermatol. 80, 115–119. [DOI] [PubMed] [Google Scholar]
- 2.Kanaoka, Y., Maekawa, A., Penrose, J. F., Austen, K. F. & Lam, B. K. (2001) J. Biol. Chem. 276, 22608–22613. [DOI] [PubMed] [Google Scholar]
- 3.Maekawa, A., Austen, K. F. & Kanaoka, Y. (2002) J. Biol. Chem. 277, 20820–20824. [DOI] [PubMed] [Google Scholar]
- 4.Davidson, A. B., Lee, T. H., Scanlon, P. D., Solway, J., McFadden, E. R., Jr., Ingram, R. H., Corey, E. J., Austen, K. F. & Drazen, J. M. (1987) Am. Rev. Respir. Dis. 135, 333–337. [DOI] [PubMed] [Google Scholar]
- 5.Griffin, M., Weiss, J. W., Leitch, A. G., McFadden, E. R., Jr., Corey, E. J., Austen, K. F. & Drazen, J. M. (1983) N. Engl. J. Med. 308, 436–439. [DOI] [PubMed] [Google Scholar]
- 6.Hamilton, A., Faiferman, I., Stober, P., Watson, R. M. & O'Byrne, P. M. (1998) J. Allergy Clin. Immunol. 102, 177–183. [DOI] [PubMed] [Google Scholar]
- 7.Chervinsky, P., Brandon, M., Zhang, J., Kundu, S., McBurney, J. & Reiss, T. F. (1998) Eur. Respir. J. 11, 1232–1239. [DOI] [PubMed] [Google Scholar]
- 8.Suissa, S., Dennis, R., Ernst, P., Sheehy, O. & Wood-Dauphinee, S. (1997) Ann. Intern. Med. 126, 177–183. [DOI] [PubMed] [Google Scholar]
- 9.Laitinen, L. A., Laitinen, A., Haahtela, T., Vilkka, V., Spur, B. W. & Lee, T. H. (1993) Lancet 341, 989–990. [DOI] [PubMed] [Google Scholar]
- 10.Minoguchi, K., Kohno, Y., Minoguchi, H., Kihara, N., Sano, Y., Yasuhara, H. & Adachi, M. (2002) Chest 121, 732–738. [DOI] [PubMed] [Google Scholar]
- 11.Henderson, W. R., Jr., Tang, L. O., Chu, S. J., Tsao, S. M., Chiang, G. K., Jones, F., Jonas, M., Pae, C., Wang, H. & Chi, E. Y. (2002) Am. J. Respir. Crit. Care Med. 165, 108–116. [DOI] [PubMed] [Google Scholar]
- 12.Lynch, K. R., O'Neill, G. P., Liu, Q., Im, D. S., Sawyer, N., Metters, K. M., Coulombe, N., Abramovitz, M., Figueroa, D. J., Zeng, Z., et al. (1999) Nature 399, 789–793. [DOI] [PubMed] [Google Scholar]
- 13.Heise, C. E., O' Dowd, B. F., Figueroa, D. J., Sawyer, N., Nguyen, T., Im, D.-S., Stocco, R., Bellefeuille, J. N., Abramovitz, M., Cheng, R., et al. (2000) J. Biol. Chem. 275, 30531–30536. [DOI] [PubMed] [Google Scholar]
- 14.Figueroa, D. J., Breyer, R. M., Defoe, S. K., Kargman, S., Daugherty, B. L., Waldburger, K., Liu, Q., Clements, M., Zeng, Z., O'Neill, G. P., et al. (2001) Am. J. Respir. Crit. Care Med. 163, 226–233. [DOI] [PubMed] [Google Scholar]
- 15.Steinke, J. W., Bradley, D., Arango, P., Crouse, C. D., Frierson, H., Kountakis, S. E., Kraft, M. & Borish, L. (2003) J. Allergy Clin. Immunol. 111, 342–349. [DOI] [PubMed] [Google Scholar]
- 16.Sarau, H. M., Ames, R. S., Chambers, J., Ellis, C., Elshourbagy, N., Foley, J. J., Schmidt, D. B., Muccitelli, R. M., Jenkins, O., Murdock, P. R., et al. (1999) Mol. Pharmacol. 56, 657–663. [DOI] [PubMed] [Google Scholar]
- 17.Nothacker, H. P., Wang, Z., Zhu, Y., Reinscheid, R. K., Lin, S. H. S. & Civelli, P. (2000) Mol. Pharmacol. 58, 1601–1608. [DOI] [PubMed] [Google Scholar]
- 18.Di Virgilio, F., Chiozzi, P., Ferrari, D., Falzoni, S., Sanz, J. M., Morelli, A., Torboli, M., Bolognesi, G. & Baricordi, O. R. (2001) Blood 97, 587–600. [DOI] [PubMed] [Google Scholar]
- 19.Mellor, E. A., Maekawa, A., Austen, K. F. & Boyce, J. A. (2001) Proc. Natl. Acad. Sci. USA 98, 7964–7969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mellor, E. A., Austen, K. F. & Boyce, J. A. (2002) J. Exp. Med. 195, 583–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ochi, H., Hirani, W. M., Yuan, Q., Friend, D., Austen, K. F. & Boyce, J. A. (1999) J. Exp. Med. 190, 267–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Price, K. S., Friend, D. S., Mellor, E. A., De Jesus, N. J., Watts, G. F. M. & Boyce, J. A. (2003) Am. J. Respir. Cell Mol. Biol. 28, 405–410. [DOI] [PubMed] [Google Scholar]
- 23.Hsieh, F. H., Lam, B. K., Penrose, J. F., Austen, K. F. & Boyce, J. A. (2001) J. Exp. Med. 193, 123–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang, L., Manji, G. A., Grenier, J. M., Al-Garawi, A., Merriam, S., Lora, J. M., Geddes, B. J., Briskin, M., DiStefano, P. S. & Bertin, J. (2002) J. Biol. Chem. 277, 29874–29880. [DOI] [PubMed] [Google Scholar]
- 25.Mellado, M., Rodriguez-Frade, J. M., Vila-Coro, A. J., Fernandez, S., Martin De Ana, A., Jones, D. R., Toran, J. L. & Martinez, A. C. (2001) EMBO J. 20, 2497–2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lova, P., Paganini, S., Sinigaglia, F., Balduini, C. & Torti, M. (2002) J. Biol. Chem. 277, 12009–12015. [DOI] [PubMed] [Google Scholar]
- 27.Sur, S., Crotty, T. B., Kephart, G. M., Hyma, B. A., Colby, T. V., Reed, C. E., Hunt, L. W. & Gleich, G. J. (1993) Am. Rev. Respir. Dis. 148, 713–719. [DOI] [PubMed] [Google Scholar]