Abstract
One of the major mechanisms for terminating the actions of catecholamines and vasoactive dietary amines is oxidation by monoamine oxidase (MAO). Smokers have been shown to have reduced levels of brain MAO, leading to speculation that MAO inhibition by tobacco smoke may underlie some of the behavioral and epidemiological features of smoking. Because smoking exposes peripheral organs as well as the brain to MAO-inhibitory compounds, we questioned whether smokers would also have reduced MAO levels in peripheral organs. Here we compared MAO B in peripheral organs in nonsmokers and smokers by using positron emission tomography and serial scans with the MAO B-specific radiotracers,l-[11C]deprenyl and deuterium-substituted l-[11C]deprenyl (l-[11C]deprenyl-D2). Binding specificity was assessed by using the deuterium isotope effect. We found that smokers have significantly reduced MAO B in peripheral organs, particularly in the heart, lungs, and kidneys, when compared with nonsmokers. Reductions ranged from 33% to 46%. Because MAO B breaks down catecholamines and other physiologically active amines, including those released by nicotine, its inhibition may alter sympathetic tone as well as central neurotransmitter activity, which could contribute to the medical consequences of smoking. In addition, although most of the emphases on the carcinogenic properties of smoke have been placed on the lungs and the upper airways, this finding highlights the fact that multiple organs in the body are also exposed to pharmacologically significant quantities of chemical compounds in tobacco smoke.
Smoking is a major public health problem affecting multiple organ systems and resulting in ≈440,000 deaths per year in the United States alone (1). Yet, we still know very little about the molecular mechanisms underlying smoking behavior and toxicity. In addition, even though tobacco smoke contains ≈4,000 chemical compounds, pharmacological studies have focused mainly on nicotine (2). We have shown that smokers have reduced levels of brain monoamine oxidase (MAO; EC 1.4.3.4) and that this is not an effect of nicotine (3–5). MAO oxidizes amines and produces hydrogen peroxide as a byproduct. It is present in virtually every organ in the body and occurs in two different subtypes, MAO A and MAO B, which are different gene products. MAO A and B have different substrate and inhibitor specificities (6). MAO A preferentially oxidizes norepinephrine and serotonin and is selectively inhibited by clorgyline (7), whereas MAO B preferentially breaks down benzylamine and phenylethylamine (PEA), and is selectively inhibited by l-deprenyl (8). Both forms oxidize dopamine and tyramine (9). The relative ratios of MAO A and B in different organs are both organ- and species-dependent, making it difficult to use animals as a model for humans (10).
Because MAO is one of the phase I oxidative enzymes (11), and its substrates include many physiologically active amines, including some of those released by nicotine, the documentation of reduced brain MAO B levels in smokers has contributed to speculation that reduced MAO B levels may account for some of the behavioral and epidemiological characteristics of smoking. For example, there is an apparent reduced rate of Parkinson's disease in smokers, suggesting that components of tobacco smoke are neuroprotective (12). In addition, there is a higher rate of smoking in psychiatric illness, including addictions to other substances, suggesting that smokers are self-medicating (13, 14). Low brain MAO in smokers has also stimulated the search for and isolation of MAO-inhibitory compounds in tobacco (15), and to the investigation of the MAO B inhibitor drug, l-deprenyl, for smoking cessation (16).
Because smoking exposes peripheral organs as well as the brain to MAO-inhibitory compounds, and because reduced MAO B in peripheral organs could potentially alter sympathetic tone and contribute to some of the physiological effects of smoking, we questioned whether smokers would also have reduced MAO in peripheral organs. Here we compared MAO B in peripheral organs in nonsmokers and smokers by using positron emission tomography (PET) and serial scans with the MAO B-specific radiotracers, l-[11C]deprenyl, and deuterium-substituted l-[11C]deprenyl (l-[11C]deprenyl-D2) (Fig. 1). Carbon-11 has a 20.4-min half life, and decays by positron emission. The 511-KeV annihilation photons arising from positron decay are detected by PET, and allow visualization of the concentration and movement of carbon-11 and other positron emitter-labeled radiotracers in a volume element of tissue (17). l-deprenyl is a mechanism-based inhibitor of MAO B. MAO-catalyzed oxidation involves the cleavage of a C–H (or the C–D bond, in the case of l-deprenyl-D2) bond on the methylene carbon of the propargyl group in the molecule, which results in the covalent attachment of l-deprenyl to the enzyme (18, 19). Because l-deprenyl is labeled with carbon-11, MAO-catalyzed oxidation results in the labeling of MAO B in vivo. MAO B was quantified in heart, lungs, kidneys, and spleen by using a three-compartment model to calculate K1, the plasma-to-organ transfer constant, which is related to blood flow and k3 or λk3, the model terms proportional to MAO B (20). Binding specificity for MAO B was assessed in different organs, based on the deuterium isotope effect. The deuterium isotope effect refers to a reduction in the rate of a reaction that occurs when a deuterium atom is substituted for hydrogen atom in a chemical bond, which is cleaved in the rate-limiting step of a reaction (21). MAO is well known to exhibit a robust deuterium isotope effect, and thus a comparison of the rate of binding of l-[11C]deprenyl and l-[11C]deprenyl-D2 provides an assessment of the specificity of the imaging method for detecting MAO B activity (22).
Fig. 1.
Structures of l-[11C]deprenyl and deuterium-substituted l-[11C]deprenyl (l-[11C]deprenyl-D2). The C–H (C–D) bond in the methylene carbon of the propargyl group is the bond that is cleaved in the rate contributing step of MAO-catalyzed oxidation.
Materials and Methods
Subjects. These studies were approved by the Institutional Review Board at Brookhaven National Laboratory, and written informed consent was obtained from each subject after the procedures had been explained. Twelve healthy smokers were recruited by newspaper advertisements and by word of mouth (see Table 1 for subject information). Exclusion criteria included medical illness that may affect monoamine levels or monoamine metabolism (such as depression or hypertension); history of drug or alcohol abuse, excluding nicotine; the current use of herbal medicines or medications that may affect monoamine levels or monoamine metabolism (such as anti-depressants and herbal remedies containing ephedra); positive urine screen for drugs of abuse. Smokers were instructed to have their last cigarette before entering the imaging laboratory. A blood sample for plasma cotinine analysis (by gas chromatography; Quest Diagnostics) was taken before the first PET scan, and a breath sample was analyzed for carbon monoxide. All twelve subjects completed both scans, and both their hearts and kidneys were visualized in the same scan for all but one of the subjects. Data from eight nonsmokers studied previously was used for comparison (20).
Table 1. Subject information.
| Nonsmokers (20) | Smokers | |
|---|---|---|
| No. of subjects | 8 | 12 |
| Age, years | 38 ± 6 | 41 ± 6 |
| Gender | 6 M/2 F | 10 M/2 F |
| Race | 7 AA/1 CA | 6 AA/6 CA |
| Cigarettes per day | 24.6 ± 9.6 | |
| Smoking years | 22 ± 7.5 | |
| Cotinine, ng/ml | 348 ± 99 | |
| CO, ppm | 28 ± 16 |
M, male; F, female. AA, African American; CA, Caucasian.
PET Scans. PET scans comparing l-[11C]deprenyl and l-[11C]deprenyl-D2 [average doses were 6.4 ± 0.9 and 5.6 ± 1.3 mCi (1 Ci = 37 GBq), respectively, with specific activity of 250 mCi/μmol at time of injection] were run on a whole-body, Siemens/CTI (Knoxville, TN) HR+ positron emission tomograph (with spatial resolution of ≈4.5-mm full width at half maximum at center of field of view) in 3D dynamic acquisition mode with 2–3 h between scans. Subjects were positioned with a goal of having both the heart and kidneys within the 15-cm axial field of view. Arms were positioned overhead, out of the field of view. Blood sampling and analysis described were used (20). Briefly, arterial samples were withdrawn every 2.5 sec for the first 2.5 min by using an automated blood sampling instrument (Ole Dich Instruments, Hvidovre, Denmark), and samples were then hand drawn every minute from 2 to 6 min, and then at 8, 10, 15, 20, 30, 45, and 60 min. Each arterial blood sample was centrifuged, the plasma was pipetted, and the C-11 was counted. Plasma samples at 1, 5, 10, 20, 30, 45, and 60 min were analyzed for l-[11C]deprenyl (or l-[11C]deprenyl-D2) by using a solid-phase extraction method (23). These values were used to correct the arterial plasma time activity curve for the presence of labeled metabolites.
In addition to the dynamic PET scans, we also performed whole-body scans with l-[11C]deprenyl on one of the nonsmokers (7.66 mCi), and one of the smokers (7.42 mCi) who had previously received the dynamic scanning protocol. These whole-body scans were done on a different day than were the dynamic scans, and provided semiquantitative images of all organs, including the brain. PET scanning was initiated 25 min after tracer injection, which is the time when the initial distribution phase of the tracer is complete and when organ accumulation plateaus, reflecting the binding of the tracer to the enzyme. A standard clinical whole-body protocol provided by the PET camera manufacturer was used by using eight bed positions of 10 min each from pelvis to brain. Data were processed by using segmented transmission attenuation correction, and iterative reconstruction and images were scaled so that they could be directly compared.
Regions of Interest. For the purpose of region identification for the l-[11C]deprenyl (or l-[11C]deprenyl-D2) scans, time frames from dynamic images taken from 0–60 min were summed. Planes were added in groups of two to obtain 16–30 planes for region of interest placement as described (20). Briefly, regions for heart, lungs, liver, kidneys, and spleen were identified on 2–3 planes, and the weighted average was obtained. Right and left lung and right and left kidney regions were averaged. For the kidneys, the region placement was made on the cortex. The regions were then projected to the dynamic scans to obtain concentration of C-11 vs. time.
Kinetic Analysis. Time-activity curves for l-[11C]deprenyl and l-[11C]deprenyl-D2 were compared to identify which organs showed the reduction in binding rate, which was characteristic of the deuterium isotope effect. PET time-activity data for l-[11C]deprenyl and l-[11C]deprenyl-D2 for these organs, as well as time-activity data from arterial plasma, were used with a three-compartment model to estimate K1, the plasma-to-organ transfer constant, which is related to blood flow, k2, which is related to the transfer of tracer from organ to plasma, and k3 and the combination model parameter, λk3, which are proportional to MAO B (20). λ is defined as K1/k2, and is independent of blood flow (24). For each organ, the residual blood volume in the organ was applied as a correction factor; model terms for the lung were also corrected for 70% air in the volume of interest (20). Spillover was minimized by fitting data using times of >1.5 min for which the blood radioactivity is substantially less than the peak value, which occurs generally at times of <1 min. We have previously used a similar model to estimate functional MAO B activity in peripheral organs (20). The isotope reduction factor is defined as the ratio of λk3(H)/λk3(D). We note that in the lungs the ratio of k3(H)/k3(D) was used to estimate the isotope reduction factor and k3(D) was used to estimate MAO B activity, because the large value of K1 in smokers precluded the estimation of λk3. The notation (H) refers to l-[11C]deprenyl, and (D) refers to l-[11C]deprenyl-D2.
Statistical Analysis. The model terms K1, k3, and λk3 for heart, lungs, kidneys, and spleen for l-[11C]deprenyl and l-[11C]deprenyl-D2 were compared by using paired samples t tests for the smokers. Values of λk3 (for heart, kidneys, and spleen) and k3 (for lungs) for l-[11C]deprenyl-D2 were then compared with the group of nonsmokers by using independent (unpaired) samples t tests. We also performed the same analysis excluding all CA subjects, to remove race as a variable. Values of λk3 were also compared for CA and AA smokers, along with smoking parameters (cigarettes per day, breath CO, and plasma cotinine). The normality assumption was examined by means of the Shapiro–Wilk test (25). P values reported are two-sided unless otherwise specified.
Results
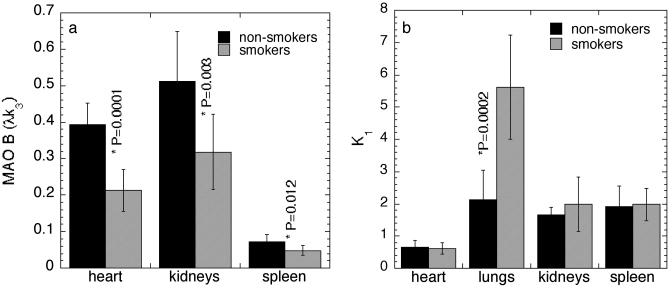
Comparison of MAO B Levels. MAO B was markedly reduced in the heart, lungs, kidneys, and spleen in the smokers relative to a group of nonsmokers studied previously (20). The reductions ranged from 33% to 46% for the model term, λk3. This difference can be seen in the whole-body images of one of the nonsmokers and one of the smokers taken 25 min after the injection of l-[11C]deprenyl (Fig. 2), and in the values of the model term, λk3, which is proportional to the number of catalytically active MAO B molecules (Fig. 3a). This marked reduction in MAO B was seen for both l-[11C]deprenyl and l-[11C]deprenyl-D2. However, the values from l-[11C]deprenyl-D2 are a more reliable index of MAO B activity because of reduced dependence on blood flow, resulting from a reduced rate of binding of the tracer to the enzyme. Anomalously high K1 in the smokers' lungs (see below) precluded the use of λk3 to estimate lung MAO B in smokers. Instead, the model term, k3, which is also proportional to MAO B activity, was compared. There was a 45% reduction in smokers relative to nonsmokers (k3 values were 0.04 ± 0.013 and 0.019 ± 0.008 ml·min–1·g–1 for nonsmokers and smokers, respectively; P = 0.0007; unpaired t value = 4.2). There were no differences in the values of the metabolite-corrected arterial plasma curve between nonsmokers and smokers for either l-deprenyl or l-[11C]deprenyl-D2.
Fig. 2.
Whole-body images of carbon-11 distribution in one of the nonsmokers and one of the smokers. These subjects were scanned with l-[11C]deprenyl, and scanning was started at 25-min post-radiotracer injection. Red is the highest radiotracer concentration on the color scale, and images are scaled so that they can be compared directly.
Fig. 3.
Model terms for nonsmokers and smokers. (a) Comparison of MAO B for different organs as assessed by the model term, λk3, in nonsmokers and smokers, which was determined from time-activity data from PET scans, and the arterial plasma radiotracer concentration after injection of l-[11C]deprenyl-D2. (b) Comparison of the plasma to tissue transfer constant, K1, in different organs for the same group of nonsmokers and smokers.
Plasma-to-Organ Transfer. Contrasting with λk3, there was no difference between nonsmokers and smokers in the average values of the plasma-to-organ transfer constant K1 for heart, kidneys, and spleen, indicating that the reductions in smokers were due to reduced MAO B, and not due to reduced blood flow (Fig. 3b). In both smokers and nonsmokers, radiotracer uptake in the liver showed a much slower accumulation of C-11 than that which was observed in other organs. Slow liver accumulation precluded the estimation of model terms, and suggests that C-11 in the liver represents nonspecific binding, which is most likely due to the accumulation of labeled metabolites (data not shown).
The lungs of smokers and nonsmokers differed markedly. The plasma-to-lung transfer constant K1 for smokers was double that of nonsmokers, indicating greater blood flow and permeability of the tracer-to-lung tissue (K1 values are 2.12 ± 0.93 and 5.62 ± 1.62 ml·min–1·g–1 for nonsmokers and smokers, respectively (P = 0.0002, unpaired t value = –4.9; Fig. 3b). Although we do not know the physiological basis for higher radiotracer transfer to lungs in smokers, others have reported anomalous radiotracer accumulation in lungs in smokers (26).
Deuterium Isotope Effect. Deuterium substitution significantly reduced the rate of radiotracer binding in the heart, kidneys, and the spleen in the smokers, indicating that MAO is involved in the rate-contributing step leading to the binding of the radiotracer in the tissue. The values of the isotope reduction factors in smokers in these organs did not differ from those in the group of nonsmokers studied (ref. 20 and Table 2). However, there was a significantly lower deuterium isotope effect for the lungs of the smokers relative to nonsmokers (Table 2). This result suggests that lung MAO B in smokers is almost completely inhibited. Although we only found a 45% reduction in k3 in the lungs of the smokers relative to the nonsmokers, this may be an underestimation. We note that low MAO B in the human lung and correspondingly low tracer uptake over a large surface area results in a weak signal and some uncertainty in quantification relative to organs like the heart and kidney. The lung would receive the largest exposure to MAO B-inhibitory compounds in smoke, which is consistent with a high degree of inhibition. Extracts of smoke have been reported to inhibit lung MAO in rats (27).
Table 2. Isotope reduction factors for nonsmokers and smokers for heart, kidneys, and spleen obtained as the ratio of λk3(H)/λk3(D) and for the lung as the ratio of k3(H)/k3(D).
| Isotope reduction factor
|
||
|---|---|---|
| Organ | Nonsmokers | Smokers |
| Heart | 3.69 ± 0.62 | 3.94 ± 1.15 |
| Lungs* | 3.79 ± 1.29 | 1.29 ± 0.74 |
| Kidneys | 5.66 ± 1.53 | 4.55 ± 1.00 |
| Spleen | 2.62 ± 0.85 | 2.14 ± 0.89 |
P = 0.0001, t = 5.1, unpaired t test
Smoking Dose. CA smokers had significantly lower heart MAO B than AA smokers by means of the independent samples t test (λk3: 0.17 ± 0.05 vs. 0.25 ± 0.033 ml ·min–1·g–1; P = 0.01) with a trend for lower MAO B in the kidneys (λk3: 0.27 ± 0.10 vs. 0.37 ± 0.09 ml·min–1·g–1; P = 0.09). However, significant reductions in MAO B in heart (P = 0.0006), kidneys (P = 0.04), and spleen (trend) remained when all CA subjects were excluded from the comparative analysis of smokers and nonsmokers. It is likely that higher MAO B in AA smokers is due to a lower number of cigarettes smoked per day than that of the CA smokers [20 ± 7 vs. 29 ± 10 cigarettes per day in self-reports; P = 0.10 (trend)], which is also reflected in lower values of expired carbon monoxide [CO: 19 ± 6 vs. 37 ± 19 ppm; P = 0.06 (trend)]. Thus, a lower exposure to MAO B-inhibitory compounds in smoke for AA smokers in this study may account for this difference.
Discussion
Enzymes that break down physiologically active chemical compounds are of particular importance in human health, because of their regulatory and protective properties. The regulatory and protective roles of MAO are well illustrated by both the effectiveness of MAO-inhibitor drugs in the treatment of depression, and by reports of serious and sometimes fatal elevations in blood pressure when individuals who are treated with nonsubtype-selective irreversible MAO-inhibitor drugs ingest foods containing the vasoactive dietary amine tyramine (for review see ref. 28). Indeed, the common warning to avoid coadministration of certain prescription and over-the-counter drugs with MAO-inhibitor drugs speaks to the importance of robust MAO activity in peripheral organs.
We have shown that human smokers have a reduction of 33–46% in MAO B in the heart, lungs, kidneys, and spleen. Reduced MAO B in peripheral organs could potentially alter sympathetic tone, and could enhance levels of MAO B-specific substrates, including endogenous substrates and dietary amines as well as those released by nicotine in tobacco smoke. For example, PEA is a specific MAO B substrate. It is produced by the decarboxylation of phenylalanine and readily crosses the blood–brain barrier. There is evidence that PEA is a neuromodulator of dopamine activity (29). Moreover, a high-affinity G protein-coupled receptor for PEA was recently discovered (30). Messenger RNA for this receptor is present in the kidney, supporting a role in blood pressure regulation and electrolyte homeostasis. The discovery of a PEA receptor raises the possibility that PEA itself may be a neurotransmitter under conditions of MAO B inhibition (30). Although circulating PEA levels are normally quite low due to oxidation by MAO B, enhanced levels of PEA are known to occur under conditions of MAO B inhibition. For example, elevated brain PEA was reported from postmortem analyses of the brains of Parkinson's patients treated with l-deprenyl (31).
MAO B-knockout mice show a tendency for lower blood pressure than do wild-type mice (32). This phenomenon has been examined in detail in MAO A- and MAO B-deficient mice, which have a phenotype characterized by elevated tissue levels of norepinephrine, serotonin, and dopamine, as well as PEA (33), and show a greater baroreceptor-mediated reduction in heart rate in response to drug-induced hypertension. This result suggests that prevention of hypertension in chronic states of catecholaminergic/indoleaminergic excess induced by MAO deficiency may be mediated by increased gain of the baroreflex. We note that epidemiological studies have documented that smokers have lower blood pressure than do nonsmokers or former smokers (34), which is consistent with the development of a compensatory mechanism to prevent hypertension.
Nicotine in tobacco smoke interacts with receptors in the brain and peripheral organs, resulting in the release of catecholamines and other physiologically active molecules (2). Both smoked and infused nicotine increase heart rate, myocardial contractility, and blood pressure. These cardiovascular effects are due mainly to activation of the sympathetic nervous system caused by nicotine-stimulated activation of nicotinic acetylcholine receptors located on peripheral postganglionic sympathetic nerve endings, as well as the adrenal medulla (35). Activation results in exocytotic release of norepinephrine, and increased levels of circulating norepinephrine and epinephrine (36). Taken together, nicotine-induced enhanced sympathetic tone and reduced peripheral MAO may both contribute to some of the central and peripheral effects of smoking. It is also possible that peripheral MAO B inhibition may be detrimental under conditions where there is compromised cardiovascular regulation. For example, in patients with Parkinson's disease, inhibition of MAO B with l-deprenyl is associated with a higher incidence of orthostatic hypotension (37), suggesting adverse effects of MAO B inhibition under conditions of autonomic dysregulation.
The consequences of reduced peripheral MAO B might be beneficial, harmful, or neutral, depending on the tissue, and whether particular exogenous substrates are present (10). As an example, MAO B catalyzes the conversion of N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) to 4-phenylpyridinium, MPP+ (38), which is toxic to nigral cells. Thus, a reduced level of MAO B in brain would afford protection from parkinsonism induced by MPTP and similar protoxins. However, a robust peripheral MAO B may reduce the brain's exposure to MPTP-like compounds by oxidizing them to charged metabolites that cannot cross the blood–brain barrier (39).
The consequences of reduced MAO in smokers, and particularly the apparent inverse relationship between smoking and the risk of Parkinson's disease, also needs to be considered from the perspective of reduced formation of the MAO byproduct, hydrogen peroxide, which is a potential source of free radicals and oxidative stress (40). There is also evidence that hydrogen peroxide formed during MAO-catalyzed oxidation of dopamine in the kidney may also play a role in cell signaling and function (41).
Although we report here that smokers also have a reduced MAO B in certain peripheral organs, it is important to point out that inhibition is partial. In addition, we were not able to measure MAO in important organs like the liver and the gut, where postmortem analyses have shown high MAO B levels (42), and thus we were not able to determine whether living smokers also have reduced MAO B in these organs. The inability to image and quantify liver MAO B is most likely because the liver is serving as a major pathway for the excretion of labeled metabolites, and thus the PET image is dominated by nonspecific binding, rather than by radiotracer binding to MAO B (20). Nonetheless, our present observation of 33–46% reduction in MAO in heart, lungs, kidneys, and spleen suggests that there may be a global inhibition involving other peripheral organs. This discovery is consistent with reports of reduced catecholamine metabolites and platelet MAO in living smokers (43).
The morbidity and mortality associated with smoking are staggering, and yet, mechanisms underlying smoking toxicity are not well understood. For example, although carbon monoxide has long been considered to be a major cardiotoxic component of smoke, a recent study (44) suggests that carbon monoxide, at the levels inhaled by the smoker, does not account for the cardiovascular effects of tobacco smoke. Because nicotine causes the release of catecholamines, both centrally and peripherally, the possibility that MAO inhibition in peripheral organs may combine with nicotine to influence local and circulating catecholamine levels and sympathetic tone in the smoker needs to be considered. In addition, although most of the emphasis on the carcinogenic properties of cigarette smoke have been placed on the lungs and the upper airways, this finding highlights the fact that pharmacologically significant quantities of chemical compounds in tobacco smoke are being delivered to various organs in the body of the smoker. Although we have only examined the effects of smoking on MAO B, this article raises the possibility that other molecular targets need to be examined to better understand the mechanisms underlying the behavioral and medical consequences of smoking.
Acknowledgments
We thank Michael Schueller for cyclotron operations, Karen Apelskog for protocol coordination, Rogelio Perez Padilla for his suggestions, and the people who volunteered for these studies. This work was carried out at Brookhaven National Laboratory under contract DE-AC02-98CH10886 with the U.S. Department of Energy. This work was supported by the Office of Biological and Environmental Research and by National Institute for Biomedical Imaging and Bioengineering Grant EB002630, National Institute on Drug Abuse Grants DA 7092-01 and DA00280, and by the Office of National Drug Control Policy.
This contribution is part of the special series of Inaugural Articles by members of the National Academy of Sciences elected on April 29, 2003.
Abbreviations: PET, positron emission tomography; MAO, monoamine oxidase; CA, Caucasian; AA, African American; PEA, phenylethylamine.
References
- 1.Centers for Disease Control (2003) Morbid. Mortal. Wkly. Rep. 52, 303–307. [Google Scholar]
- 2.Benowitz N. L. (1988) N. Engl. J. Med. 319, 1318–1331. [DOI] [PubMed] [Google Scholar]
- 3.Fowler, J. S., Volkow, N. D., Wang, G.-J., Pappas, N., Logan, J., Shea, C., Alexoff, D., MacGregor, R. R., Schlyer, D. J., Zezulkova, I., et al. (1996) Proc. Natl. Acad. Sci. USA 93, 14065–14069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fowler, J. S., Volkow, N. D., Wang, G.-J., Pappas, N., Logan, J., MacGregor, R., Alexoff, D., Shea, C., Schlyer, D., Wolf, A. P., et al. (1996) Nature 379, 733–736. [DOI] [PubMed] [Google Scholar]
- 5.Fowler, J. S., Volkow, N. D., Logan, J., Pappas, N., King, P., MacGregor, R., Shea, C., Garza, V. & Gatley, S. J. (1998) Life Sci. 63, 19–23. [DOI] [PubMed] [Google Scholar]
- 6.Shih, J. C., Chen, K. & Ridd, M. J. (1999) Annu. Rev. Neurosci. 22, 197–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnston, J. P. (1998) Biochem. Pharmacol. 17, 1285–1297. [DOI] [PubMed] [Google Scholar]
- 8.Knoll, J. & Magyar, K. (1972) Adv. Biochem. Psychopharmacol. 5, 393–408. [PubMed] [Google Scholar]
- 9.Youdim, M. D. H. & Riederer, P. (1993) J. Neural Transm. 91, 181–195. [DOI] [PubMed] [Google Scholar]
- 10.Inoue, H., Castagnoli, K., Van Der Schyf, C., Mabic, S., Igarashi, K. & Castagnoli, N., Jr. (1999) J. Pharmacol. Exp. Ther. 291, 856–864. [PubMed] [Google Scholar]
- 11.Strolin Benedetti, M. &Tipton, K. F. (1998) J. Neural Transm. Suppl. 52, 149–171. [DOI] [PubMed] [Google Scholar]
- 12.Morens, D. M., Grandinetti, A., Reed, D., White, L. R. & Ross, G. W. (1995) Neurology 45, 1041–1051. [DOI] [PubMed] [Google Scholar]
- 13.Hughes, J. R., Hatsukami, D. K., Mitchell, J. E. & Dahlgren, L. A. (1986) Am. J. Psychiatry 143, 993–997. [DOI] [PubMed] [Google Scholar]
- 14.Henningfield, J. E., Clayton, R. & Pollen, W. (1990) Br. J. Addict. 85, 279–282. [DOI] [PubMed] [Google Scholar]
- 15.Khalil, A. A., Steyn, S. & Castagnoli, N., Jr. (2000) Chem. Res. Toxicol. 13, 31–35. [DOI] [PubMed] [Google Scholar]
- 16.George, T. P., Vessicchio, J. C., Termine, A., Jatlow, P. I., Kosten, T. R. & O'Malley, S. S. (2003) Biol. Psychiatry 53, 136–143. [DOI] [PubMed] [Google Scholar]
- 17.Phelps, M. E. (2000) Proc. Natl. Acad. Sci. USA 97, 9226–9233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maycock, A. L., Abeles, R. H., Salach, J. I. & Singer, T. P. (1976) Biochemistry 15, 114–125. [DOI] [PubMed] [Google Scholar]
- 19.Fowler, J. S., Wolf, A. P., MacGregor, R. R., Dewey, S. L., Logan, J., Schlyer, D. J. & Langstrom, B. (1988) J. Neurochem. 51, 1524–1534. [DOI] [PubMed] [Google Scholar]
- 20.Fowler, J. S., Logan, J., Wang, G.-J., Volkow, N. D., Zhu, W., Franceschi, D., Pappas, N., Ferrieri, R., Shea, C., Garza, V., et al. (2002) J. Nucl. Med. 43, 1331–1338. [PubMed] [Google Scholar]
- 21.Belleau, B. & Moran, J. (1963) Ann. N.Y. Acad. Sci. 107, 822–839. [DOI] [PubMed] [Google Scholar]
- 22.Fowler, J. S., Wang, G.-J., Logan, J., Xie, S., Volkow, N. D., MacGregor, R. R., Schlyer, D. J., Pappas, N., Alexoff, D. L., Patlak, C., et al. (1995) J. Nucl. Med. 36, 1255–1262. [PubMed] [Google Scholar]
- 23.Alexoff, D. L., Shea, C., Fowler, J. S., King, P., Gatley, S. J., Schlyer, D. J. & Wolf, A. P. (1995) Nucl. Med. Biol. 22, 893–904. [DOI] [PubMed] [Google Scholar]
- 24.Logan, J., Dewey, S. L., Wolf, A. P., Fowler, J. S., Brodie, J. D., Angrist, B., Volkow, N. D. & Gatley, S. J. (1991) Synapse 9, 195–207. [DOI] [PubMed] [Google Scholar]
- 25.Shapiro, S. S. & Wilk, M. B. (1965) Biometrika 52, 591–611. [Google Scholar]
- 26.Kagaya, A. (1992) Kaku Igaku 29, 1099–1106. [PubMed] [Google Scholar]
- 27.Yu, P. H. & Boulton, A. A. (1987) Life Sci. 41, 675–682. [DOI] [PubMed] [Google Scholar]
- 28.Caldecott-Hazard, S. & Schneider, L. S. (1992) Synapse 10, 141–168. [DOI] [PubMed] [Google Scholar]
- 29.Paterson, I. A., Juorio, A. V. & Boulton, A. A. (1990) J. Neurochem. 55, 1827–1837. [DOI] [PubMed] [Google Scholar]
- 30.Borowsky, B., Adham, N., Jones, K. A., Raddatz, R., Artymyshyn, R., Ogozalek, K. L., Durkin, M. M., Lakhlani, P. P., Bonini, J. A., Pathirana, S., et al. (2001) Proc. Natl. Acad. Sci. USA 98, 8966–8971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Riederer, P. & Youdim, M. B. H. (1986) J. Neurochem. 46, 1359–1365. [DOI] [PubMed] [Google Scholar]
- 32.Scremin, O. U., Holschneider D. P., Chen, K., Li, M. G. & Shih, J. C. (1999) Brain Res. 824, 36–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Holschneider, D. P., Scremin, O. U., Chialvo, D. R., Chen, K. & Shih, J. C. (2002) Am. J. Physiol. 282, H1751–H1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Green, M. S., Jucha, E. & Luz, Y. (1986) Am. Heart J. 111, 932–940. [DOI] [PubMed] [Google Scholar]
- 35.Haass, M. & Kubler, W. (1997) Cardiovasc. Drugs Ther. 10, 657–665. [DOI] [PubMed] [Google Scholar]
- 36.Walker, J. F., Collins, L. C., Rowell, P. P., Goldsmith, L. J., Moffatt, R. J. & Stamford, B. A. (1999) Nicotine Tob. Res. 1, 365–370. [DOI] [PubMed] [Google Scholar]
- 37.Turkka, J., Suominen, K., Tolonen, U., Sotaniemi, K. & Myllyla, V. V. (1997) Neurology 48, 662–667. [DOI] [PubMed] [Google Scholar]
- 38.Heikkila, R. E., Manzino, L. Cabbat, F. S. & Duvoisin, R. C. (1984) Nature 311, 467–469. [DOI] [PubMed] [Google Scholar]
- 39.Kalaria, R. N. & Harik, S. I. (1987) J. Neurochem. 49, 856–864. [DOI] [PubMed] [Google Scholar]
- 40.Cohen, G., Farooqui, R. & Kesler, N. (1997) Proc. Natl. Acad. Sci. USA 94, 4890–4894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vindis, C., Seguelas, M. H., Lanier, S., Parini, A. & Cambon, C. (2001) Kidney Int. 59, 76–86. [DOI] [PubMed] [Google Scholar]
- 42.Saura, J., Nadal, E., van den Berg, B., Vila, M., Bombi, J. A. & Mahy, N. (1996) Life Sci. 59, 1341–1349. [DOI] [PubMed] [Google Scholar]
- 43.Berlin, I., Said, S., Spreux-Varoquaux, O., Olivares, R., Launay, J. M. & Puech, A. J. (1995) Biol. Psychiatry 33, 756–761. [DOI] [PubMed] [Google Scholar]
- 44.Zevin, S., Saunders, S., Gourlay, S. G., Jacob, P. & Benowitz, N. L. (2001) J. Am. Coll. Cardiol. 38, 1633–1638. [DOI] [PubMed] [Google Scholar]