Abstract
Erythropoietin (EPO), well known for its role in stimulation of erythropoiesis, has recently been shown to have a dramatic neuroprotective effect in animal models of cerebral ischemia, mechanical trauma of the nervous system, and excitotoxins, mainly by reducing apoptosis. We studied the effect of single systemic administration of recombinant human EPO (rhEPO) on left ventricular (LV) size and function in rats during 8 weeks after the induction of a myocardial infarction (MI) by permanent ligation of the left descending coronary artery. We found that an i.p. injection of 3,000 units/kg of rhEPO immediately after the coronary artery ligation resulted, 24 h later, in a 50% reduction of apoptosis in the myocardial area at risk. Eight weeks after the induction of MI, rats treated with rhEPO had an infarct size 15–25% of the size of that in untreated animals. The reduction in myocardial damage was accompanied by reductions in LV size and functional decline as measured by repeated echocardiography. Thus, a single dose of rhEPO administered around the time of acute, sustained coronary insufficiency merits consideration with respect to its therapeutic potential to limit the extent of resultant MI and contractile dysfunction.
Erythropoietin (EPO), a cytokine produced by the adult kidney, is a well known hematopoietic factor. EPO receptors (EPO-Rs) are expressed in adult bone marrow and spleen and are activated by hypoxia (1). Whether EPO-Rs are present in nonhematopoietic tissues is less certain. The predominant opinion is that the expression of EPO-Rs in nonhematopoietic tissues is limited to the fetal stage of development (2). Although some studies failed to detect EPO-R transcripts in the brain, kidney, liver, or heart of adult mice (3), others have reported an intensive immunoreactivity for EPO-Rs in many medium and large neurons of adult rat brain (4). Moreover, a weak EPO-R immunoreactivity of human brain was amplified by hypoxia (5). Recently, EPO-Rs have also been identified in the adult retina of mice (6). Although EPO-Rs have not been identified in adult hearts, their presence during embryogenesis is critical for cardiac development (7).
Recombinant human EPO (rhEPO) is widely used for the treatment of anemia occurring in the context of surgery, cancer, HIV, kidney failure, etc. (8). Recently, rhEPO has been shown to have a dramatic neuroprotective effect in animal models of cerebral ischemia and mechanical trauma of the nervous system, and in response to excitotoxins. A single intracerebroventricular injection and, more importantly, systemic administration of rhEPO have resulted in a 50–75% reduction in brain injury induced by the focal ischemia (4). A reduction of apoptosis is a mechanism involved in this neuroprotective effect of rhEPO (9, 10).
We hypothesized that the protective effect of systemic rhEPO administration that resulted in improvement of brain cell survival after cerebral ischemia would also occur in the ischemic heart model. Specifically, we studied the effect of a single systemic administration of rhEPO on cardiac performance and the size/structure of the heart during 8 weeks after permanent ligation of the left anterior descending coronary artery in rats. We hypothesized that rhEPO would reduce apoptosis and final infarct size, and that these effects would be accompanied by a reduction in left ventricular (LV) contractile performance decline and a reduction in the extent of post myocardial infarction (MI) LV dilatation, wall thinning, and heart failure.
Methods
Animals and Experimental Design. Eighty six male Sprague–Dawley rats, 3–4 months of age (body weight 404 ± 11.6 g), were housed and studied in conformance with the NIH Guide for the Care and Use of Laboratory Animals, Manual 3040-2 (1999), with institutional Animal Care and Use Committee approval. After baseline echocardiography, 74 animals were randomly divided into experimental (n = 54) or sham (SH) (n = 20) groups and, under anesthesia by i.p. injection of sodium pentobarbital (50 mg/kg), subjected to ligation of the paraconal interventricular branch of the left coronary artery (corresponding to the left anterior descending coronary artery in humans) or to SH operation, similar to that described (11). Half of the animals in the experimental and half in the SH group received a single injection of rhEPO (3,000 units/kg in 0.3 ml of saline, i.p.) immediately (<5 min) after surgery. Other rats received a single i.p. injection of 0.3 ml of saline at the same time point. Therefore, the experimental design consisted of four groups of rats: SH not treated (SHnT), SH treated with rhEPO (SHT), MI not treated (MInT), and MI treated with rhEPO (MIT). Seven animals from the MIT group and seven animals from the MInT group were killed 24 h after surgery, and their hearts were harvested for appropriate histochemical staining to assess the early effect of rhEPO treatment on the extent of post-MI apoptosis. In the remainder of the operated animals, LV function was assessed repeatedly by Doppler echocardiography at weeks 1, 4, and 8 after surgery, at which time all animals were killed by a bolus injection of KCl after general anesthesia with sodium pentobarbital, and their hearts were harvested for histological analyses. To test the erythropoietic effect of EPO in our experimental setting, 3,000 units/kg rhEPO (in 0.3 ml of saline, i.p.) was injected into an additional subset of six naive rats. The same number of animals was injected with 0.3 ml of saline. Blood (0.5 ml) was collected from the jugular vein under pentobarbital anesthesia just before treatment and at 2, 4, 7, 10, 14, and 21 days after treatment, and hematocrit was measured in each aliquot in triplicate.
Materials. All experiments were performed by using Epoetin Alfa (Procrit, Amgen Biologicals). The rhEPO, a human 165-aa glycoprotein, manufactured by using recombinant DNA technology, contains the same amino acid sequence as natural EPO and possesses the same biological activity. It is ≈80% homologous to rodent EPO, and it has been shown to be biologically active in rodents with respect to erythropoietic function (12, 13).
Doppler Echocardiography. Cardiac function was assessed by echocardiography (HP Sonos 5500 equipped with a 12-Mhz phase array linear transducer, S12, allowing a 150 maximal sweep rate) under general anesthesia with pentobarbital sodium (30 mg/kg i.p.) as described (14). Parasternal long axis views were obtained and recorded, ensuring that the mitral and aortic valves and the apex were visualized. Short axis views were recorded at the level of mid-papillary muscles. Both 2D and motion (M)-mode views were recorded at the same level. Measurements of LV end-systolic dimension (LVESD) and end-diastolic dimension (LVEDD), both in short and long axis views, were made from M-mode. Endocardial area tracings using the leading edge method were performed in the 2D mode (short and long axis views) from digital images captured on cineloop to calculate end-diastolic and end-systolic LV areas (LVEDA and LVESA). LV end-diastolic volume (LVEDV) and LV end-systolic volume (LVESV) were calculated by a modified Simpson's method from the long axis view. LV fractional shortening (LVFS %) in the long axis view was calculated as LVFS = (LVEDD – LVESD)/LVEDD × 100, and the LV fractional area change (LVFAC %) in the short axis view was calculated as LVFAC = (LVEDA – LVESA)/LVEDA × 100. LV ejection fraction (LVEF %) was then derived as LVEF = (LVEDV – LVESV)/LVEDV × 100. All measurements were made by one observer who was blinded with respect to the identity of the tracings. All measurements were averaged over three to five consecutive cardiac cycles. The reproducibility of measurements was assessed at baseline by two sets of measurements in 10 randomly selected rats. The repeated measures variability did not exceed ±5%.
Infarct Size Measurement. Hearts were excised and placed in 10% phosphate-buffered formalin. The gross infarct size was estimated in the intact heart by comparison of the length and width of the MI and LV areas. Then, the fixed tissue was embedded in paraffin and serially cut from the apex to the level just below coronary artery ligation site. Transverse 5-μm-thick sections were cut at 500-μm distances, such that 14–18 sections were obtained from each heart. Sections were stained with hematoxylin/eosin and azan, and morphological analysis was performed by computerized video imaging using an Axioplan microscope (Zeiss) and nih image software. The myocardial infarct size of each section was calculated as the ratio of infarction area to the area of total LV section (area method) and as the average of ratios of the outer infarction length to the outer LV circumference and the inner infarction length to the inner LV circumference (perimeter method). The infarct size of all sections for both area and perimeter methods was averaged and expressed as the percentage MI size for each heart.
Assessment of Apoptosis. Twenty-four hours after coronary artery ligation or SH operation, under general anesthesia with pentobarbital sodium (50 mg/kg i.p.), 2 ml of 5% Evans blue was injected into the right ventricular chamber via the right jugular vein. The rats were killed by a bolus injection of 4 ml of 0.5 M KCl, and the hearts were removed, rapidly rinsed in PBS, and snap-frozen in liquid nitrogen. Serial, 6-μm-thick cryostat sections were prepared and subsequently processed for tetrazolium chloride and terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling (TUNEL) staining. The well perfused myocardium outlined in blue was separated from the nonperfused part. The parts unstained by Evans blue containing a combination of dead tissue and underperfused but viable myocardium [area at risk (AAR)] were incubated for 20 min in tetrazolium chloride then transferred in 4% paraformaldehyde. In all resulting sections, the AAR of myocardial tissue was stained in red, although dead tissue remained white (15). The AAR was further subjected to TUNEL staining for detection of apoptotic cells by the nick-end labeling method, using a commercially available kit (Loche, Minneapolis) as directed by the manufacturer.
Slides were examined by light microscopy. In each section, the number of cardiomyocytes and the number of TUNEL-positive cardiomyocyte nuclei were counted and totaled in 10 randomly selected fields of the AAR at ×400 amplification (16). Only nuclei that were clearly located in cardiomyocytes were counted.
Statistical Analyses. After the baseline sonographic parameters were measured and analyzed, animals in which any of the indices exceeded 2 SD of the average for all animals were excluded from further analyses. Results are expressed as mean ± SEM. Sonographic indices of functional assessment at each time point are expressed as the percentage change from the baseline (measurements taken before surgery) and, where appropriate, adjusted for body mass. Statistical significance of differences among groups with regard to changes of these indices over time was determined by using ANOVA for repeated measurements, specifically noting group × time interactions. A post hoc pair comparison between MInT and MIT groups was conducted for data from weeks 1 and 8. Statistical significance of differences among groups with regard to infarct size or apoptosis was determined by using Student's t test. Statistical significance was assumed at P < 0.05.
Results
Mortality and Final Number of Animals. The total mortality among rats subjected to coronary ligation was 40.8% (33.9% died perioperatively, i.e., <12 h after surgery). The total mortality among SH operated animals was 21%, the majority of which (15.8%) also died perioperatively. There were no differences in mortality (total or perioperative) between animals that received rhEPO injection and untreated animals. After results of baseline testing were analyzed, it was determined that in three animals the LV end-systolic dimensions exceeded the average for all animals by >2 SD. The results of these three animals were discarded from further analyses. Thus, the final count of rats after 8 weeks of functional assessment was: SHnT, 7; SHT, 7; MInT, 9; and MIT, 8. Of 14 rats used for assessment of apoptosis, 1 died in each group, resulting in a final number of 6 each in MIT and MInT groups. There were no mortalities in the group of 12 rats subjected to repeated measurements of hematocrit.
The Effect of EPO on Hematocrit. The effect of a single injection of 3,000 units/kg rhEPO on the hematocrit of healthy rats is presented in Table 1. The average changes in hematocrit in rats treated with rhEPO did not differ from those receiving vehicle (P > 0.05, ANOVA for repeated measurements). The slight (2.6% and 4.7%) increases of hematocrit in EPO-treated rats on days 14 and 21, respectively, were not statistically significant.
Table 1. Serial measurements of hematocrit after a single rhEPO or saline injection (mean ± SE).
| Before | Day 2 | Day 4 | Day 7 | Day 10 | Day 14 | Day 21 | |
|---|---|---|---|---|---|---|---|
| Saline (6) | 44.8 ± 0.4 | 43.5 ± 0.7 | 42.4 ± 0.7 | 41.5 ± 0.4 | 42.7 ± 0.9 | 42.3 ± 1.1 | 42.6 ± 1.5 |
| EPO (6) | 45.6 ± 0.9 | 43.5 ± 0.9 | 41.1 ± 1.1 | 40.4 ± 1.2 | 43.4 ± 0.7 | 43.4 ± 0.8 | 44.6 ± 0.9 |
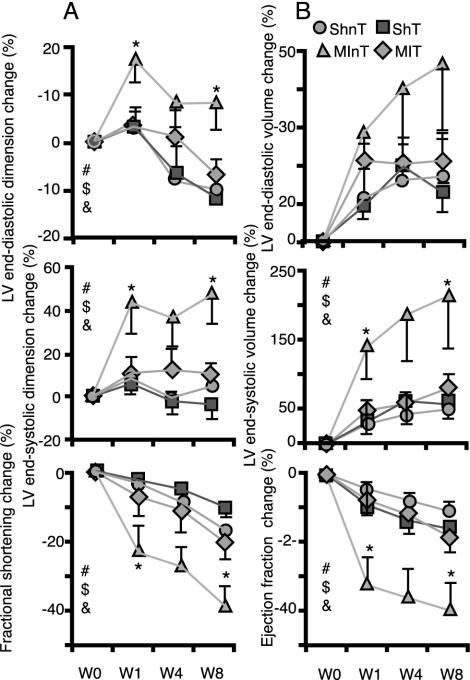
Doppler Echocardiography. The baseline data of the echo measurements of LV size and function in four groups of rats before surgery and rhEPO treatment are summarized in Table 3, which is published as supporting information on the PNAS web site, www.pnas.org. There were no statistical differences among all groups for any measured parameter. Fig. 1 illustrates the average changes in several indices of LV size (dimension and volume) and LV performance (fractional shortening and ejection fraction) during 8 weeks after coronary artery ligation or SH operation in rats treated or not treated with rhEPO. Table 2 lists the average changes for other echocardiographic measurements (LV dimensions, area, fractional shortening, and fractional area change). All LV indices revealed the same pattern of change. Treatment of SH-operated animals with rhEPO did not affect the direction or magnitude of changes during 8 weeks after surgery relative to untreated SH animals. In nontreated, ligated animals (MInT) the indices of LV size showed a gradual enlargement with time both at end-systole and end-diastole, and the pattern of the change was significantly different from that in SHnT group, indicating the marked occurrence of LV remodeling. The indices of LV performance in the MInT group showed a steeper decline in function with time compared with the SH group. For example, during 8 weeks after ligation of coronary artery (Table 2), the LVEDA increased by 38.6 ± 18.4%, the LVESA increased by 180.8 ± 88.7%, and the LVFAC fell by 37.3 ± 7.6% in the MInT group, whereas for the same period in SHnT, these indices changed by only 0.15 ± 6.6%, 23.4 ± 8.4%, and –16.1 ± 1.7% for LVEDA, LVESA, and LVFAC, respectively (P < 0.05 for each). In contrast to MInT rats, in rats treated with rhEPO after coronary ligation (MIT group), the indices of LV size and performance did not statistically differ from the SHnT group. For example, after 8 weeks, the LVEDA actually decreased by 7 ± 5.7%, the LVESA increased by only 49.4 ± 29%, and the LVFAC fell 20.5 ± 7.3% in the MIT group (Table 2). Moreover, the pattern and magnitude of changes reflecting the extent of LV remodeling and functional decline were significantly less pronounced in the MIT than in the MInT group. For most of the indices, the post hoc pair comparison revealed that the MIT and MInT groups were statistically different at week 8. In fact, for many indices, e.g., end-diastolic and end-systolic dimensions and fractional shortening change, the difference between treated and nontreated MI groups achieved statistical significance at the first week after surgery (Fig. 1).
Fig. 1.
Changes in echocardiographic indices of LV size (dimensions or volume) and function (fractional shortening or ejection fraction) during 8 weeks after coronary ligation or SH operation in rhEPO-treated and untreated rats. All indices are derived from images obtained from the long axis view in M-mode (A) or 2D mode echo (B). All indices are adjusted for body mass and expressed as the percentage change from the baseline values (see Table 3). Statistically significant (P < 0.05) group × time interactions (ANOVA for repeated measurements) are indicated by the following: #, all groups; $, MInT × MIT; &, SHnT × MInT. *, Significant (P < 0.05 difference post hoc comparison) between MInT and MIT groups at weeks 1 and 8.
Table 2. Change in body mass adjusted echocardiographic short axis indices of LV size, and LV function during 8 weeks after coronary ligation or SH operation in rhEPO-treated and control rats (%, mean ± SEM).
| Mode | Indices | Groups | Week 1 | Week 4 | Week 8 |
|---|---|---|---|---|---|
| M | LVEDD | SHnT (7)* | 6.5 ± 3.9 | -6.8 ± 2.4 | -5.9 ± 5.5 |
| SHT (7) | 8.0 ± 3.1 | -6.6 ± 3.7 | -11.5 ± 4.3 | ||
| MInT (9)*† | 21.0 ± 6.9 | 9.4 ± 6.0 | 13.2 ± 6.8 | ||
| MIT (8)* | 9.7 ± 4.4 | 3.4 ± 6.0 | -4.6 ± 3.2‡ | ||
| M | LVESD | SHnT (7)† | 8.9 ± 4.8 | -1.4 ± 3.2 | 6.4 ± 5.6 |
| SHT (7) | 13.5 ± 3.3 | 0.5 ± 3.5 | -1.5 ± 3.7 | ||
| MInT (9)*† | 50.4 ± 19.0 | 44.9 ± 18.3 | 56.1 ± 17.7 | ||
| MIT (8)* | 14.8 ± 13.7 | 15.4 ± 14.1 | 12.9 ± 10.2‡ | ||
| M | LVFS | SHnT (7)† | -2.3 ± 2.7 | -6.3 ± 3.1 | -14.3 ± 2.7 |
| SHT (7) | -5.2 ± 3.1 | -8.7 ± 2.5 | -12.6 ± 2.8 | ||
| MInT (9)*† | -22.9 ± 8.6 | -31.9 ± 9.5 | -38.8 ± 7.6 | ||
| MIT (8)* | -1.7 ± 6.9‡ | -9.1 ± 7.6 | -17.8 ± 7.3‡ | ||
| 2D | LVEDA | SHnT (7)† | 14.7 ± 6.3 | -4.0 ± 6.3 | -0.1 ± 6.6 |
| SHT (7) | 9.6 ± 3.0 | -5.9 ± 4.6 | -11.7 ± 6.0 | ||
| MInT (9)*† | 35.7 ± 14.5 | 41.7 ± 20.3 | 38.6 ± 18.4 | ||
| MIT (8)* | 2.0 ± 3.9‡ | -1.1 ± 6.9 | -7.0 ± 5.7‡ | ||
| 2D | LVESA | SHnT (7)† | 28.1 ± 11.1 | 12.1 ± 7.0 | 28.1 ± 10.7 |
| SHT (7) | 33.3 ± 8.0 | 19.8 ± 5.3 | 23.4 ± 8.4 | ||
| MInT (9)*† | 155.0 ± 76.8 | 193.4 ± 95.2 | 180.8 ± 88.7 | ||
| MIT (8)* | 43.4 ± 27.7 | 44.4 ± 26.8 | 49.4 ± 29.0 | ||
| 2D | LVFAC | SHnT (7)† | -4.0 ± 2.4 | -7.6 ± 1.4 | -13.2 ± 2.2 |
| SHT (7) | -7.8 ± 2.0 | -11.0 ± 1.3 | -16.1 ± 1.7 | ||
| MInT (9)† | -30.3 ± 9.4 | -34.1 ± 8.0 | -37.3 ± 7.6 | ||
| MIT (8) | -13.5 ± 7.9 | -15.6 ± 7.3 | -20.5 ± 7.3‡ |
Significant (P < 0.05) group × time interaction (ANOVA for repeated measurements): *, for comparison of SHnT and MinT; †, for comparison of MInT and MIT. ‡, Significant (P < 0.05) differences between the MInT and MIT groups at week 1 or week 8 (post hoc pair comparison).
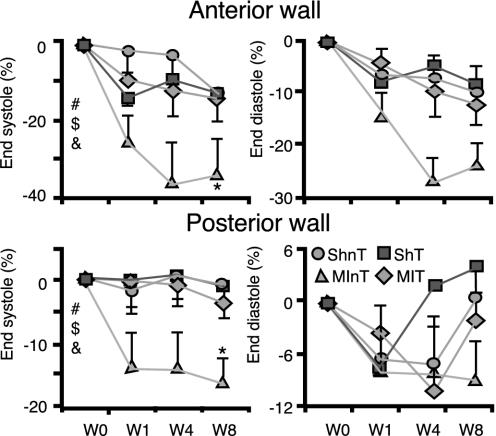
The changes in LV anterior and posterior wall thickness are presented in Fig. 2. Both the anterior and posterior LV walls at end-systole showed a statistically significant trend to become progressively thinner after surgery in the MInT group compared with the SH or MIT groups. For instance, at 8 weeks after surgery, the end-systolic posterior wall thickness was reduced by 16.6 ± 4%, 3.8 ± 2.3%, and 1 ± 4.1% in the MInT, MIT, and SHnT groups, respectively. The end-systolic thickness of the anterior wall was reduced by 34.3 ± 9.7%, 14.2 ± 5.9%, and 13.2 ± 8.4% in the MInT, MIT, and SHnT groups, respectively. The relative thinning of the LV walls in the MInT group at end-systole is compatible with the marked decline in contractile function in this group. Neither the anterior nor the posterior wall thicknesses at end-diastole in the MIT group differed from SH.
Fig. 2.
Changes in cardiac wall thickness during 8 weeks after coronary ligation or SH operation in rhEPO-treated and untreated rats. Indices are derived from M-mode echo and expressed as a percentage change from baseline values (see Table 3). All symbols are the same as in Fig. 1.
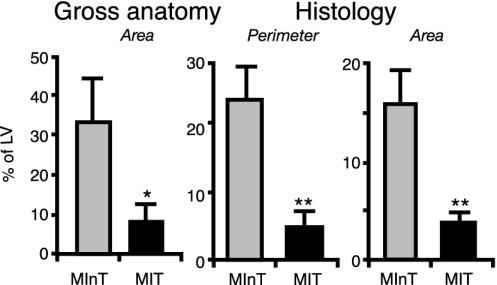
Infarct Size. The average infarct size, expressed as a percentage of LV, in the MInT and MIT groups is presented in Fig. 3, calculated as the gross infarct size (measured on the whole hearts) or on histological slides by the perimeter and area methods. Regardless of the technique used to estimate the MI size, the average MI size (expressed as a percentage of LV) in the MIT group was 15–25% of that in the MInT group. For example, as measured by the perimeter technique, the average MI size was 23.5 ± 4.7% in the MInT group and only 4.9 ± 2.4% in the MIT group (P < 0.01).
Fig. 3.
MI size 8 weeks after ligation of the coronary artery in untreated rats and in rats treated with rhEPO. *, P < 0.05; **, P < 0.01 (Student's t test).
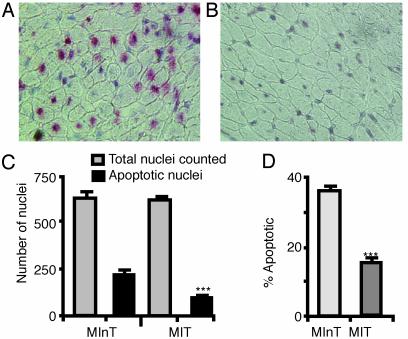
Estimation of Apoptosis in the AAR. Fig. 4 A and B illustrates the TUNEL staining of representative histological slides of comparable AARs in hearts from the MInT and MIT groups, respectively, at 24 h after coronary artery ligation. More apoptotic nuclei are clearly observed in the untreated heart (A). Fig. 4C shows the average number of apoptotic nuclei in the area at risk of treated and untreated hearts. In the MIT group, only 15.3 ± 1.2% nuclei were TUNEL positive compared with 35.6 ± 1.2% in the MInT group (P < 0.001) (Fig. 4D).
Fig. 4.
Representative examples of TUNEL staining in the AAR of myocardium 24 h after coronary ligation in rats with (B) and without (A) rhEPO treatment (amplification ×400). (C) The average number of TUNEL-positive nuclei. (D) The percentage of TUNEL-positive nuclei in hearts from coronary-ligated rats with or without rhEPO treatment. ***, P < 0.001 (Student's t test).
Discussion
The present results show that a single treatment with rhEPO immediately after permanent coronary ligation in rats results in a dramatic reduction in the extent of MI, and that this is accompanied by preserved myocardial structure and function. Twenty-four hours after coronary ligation, the number of apoptotic myocytes in the AAR in rhEPO-treated animals was reduced to less than half that of nontreated, coronary artery-ligated rats. In rhEPO-treated rats, the size of the MIs measured 8 weeks after coronary ligation was on average 15–25% of that in untreated animals. The functional decline (i.e., 40% reduction in fractional shortening measured by repeated echocardiography) in the coronary artery-ligated, untreated rats occurred in the context of a dilated, thin-walled heart. The LV remodeling and LV performance declines were significantly less pronounced in animals treated with rhEPO than in untreated animals. LV performance in rhEPO-treated rats, in fact, did not differ from SH-operated rats.
There are several known properties of EPO that might be considered as potential factors involved in the cardioprotective effects observed in the present study. The traditional and most well known property of exogenous EPO is to stimulate erythropoiesis (1). Presently, rhEPO is an established therapy for anemia of different etiologies (8). From this perspective, a benefit of increasing the oxygen-carrying capacity of blood by increasing the red blood cell mass by blood transfusion had been reported in elderly patients with acute MI, but only in cases with severe anemia; in patients with hematocrit >33%, the outcome was not affected by blood transfusion (17). A single dose of rhEPO, like that used in the present study, increases the number of circulating reticulocytes by days 3–4, which reaches a maximum level by days 8–11, and returns to baseline level by day 22 (18, 19). Although it is plausible to speculate that an increase in oxygen-carrying capacity might facilitate the surviving of myocytes after coronary ligation, it is an unlikely mechanism to reduce early apoptosis at 24 h and infarct expansion. Cell death and infarct expansion are occurring mainly, although not exclusively, during the first 3 days after ischemic insult, i.e., well before the expected maximum increase in red cell mass in response to EPO occurring. Furthermore, in our experiment, the change of hematocrit over time after a single dose of rhEPO did not differ from that of the injection of saline.
Another attribute of EPO that might be responsible for reduction of LV structural damage and functional decline after MI is its angiogenic effect. It has been shown that rhEPO added to the culture of endocardial endothelial cells significantly accelerates the naturally occurring capillary outgrowth, and its effectiveness is comparable with that of vascular endothelial growth factor (20). The naturally occurring angiogenesis in that model takes place in 14 ± 7 days, and rhEPO treatment accelerates the process by 12–24 h. Although this timing does not exclude stimulation of angiogenesis by rhEPO from consideration as a contributing cardioprotective mechanism at the late stages of LV remodeling, it makes it unlikely that angiogenesis is the main mechanism responsible for the early reduction of apoptosis. Although EPO-induced neovascularization is unlikely to occur during the first 24 h after coronary ligation, rhEPO is known to increase nitric oxide availability (21). Thus, it is conceivable that nitric oxide-associated vasodilatation and facilitated effective collateral circulation might reduce the early infarct size.
The use of stem cells of various origins in the treatment of MI and heart failure is potentially promising and is being widely explored (22). Some therapeutic approaches have capitalized on a perceived ability of stem cells to migrate to the hypoxic area of myocardium. The ability of rhEPO to stimulate the proliferation of the neural and endothelial progenitor cells has been proven in this regard (23, 24). Thus, the potential of rhEPO to stimulate the development and to mobilize the immature, nondifferentiated cells into ischemic area of myocardium is definitely a possible mechanism underlying the effect of rhEPO that we have observed.
rhEPO also reportedly possesses antiinflammatory properties. In the study on rats, systemic pretreatment with rhEPO reduced the number of inflammatory cells associated with injury in the brain tissue (4). However, in a study in tissue culture, rhEPO seemed to have little effect on the microglial proinflammatory activity (9), which suggests that the reduction of inflammation observed in vivo is a secondary, indirect effect of rhEPO, possibly related to its antiapoptotic property.
The most probable cardioprotective factor of rhEPO is its ability to reduce or prevent cell death. The present study shows that a single dose of rhEPO markedly reduces the extent of apoptosis in cardiac cells within the AAR for MI after coronary ligation. The present demonstration of the protective effect of rhEPO with respect to an ischemic insult is strikingly similar to the dramatic neuroprotective effects of a systemic application of rhEPO (4). EPO also had been shown to inhibit the apoptosis induced by serum deprivation or kainic acid exposure in microglial cells (9) and in neuronal cells in culture (10). In vivo, systemic administration of rhEPO abolished the appearance of TUNEL-positive cells in the brain tissue of rats after middle-cerebral artery occlusion (10) or in spinal motoneurones of rabbits after ischemic assault (25). All of these previous observations in neuronal tissue are consistent with our findings in the experimental myocardial infarct model in rats in the present study, and we conclude that the early antiapoptotic effect of rhEPO is likely a main mechanism responsible for reduction of MI size and attenuation of the subsequent functional decline. In the model of permanent ligation of coronary artery, rhEPO obviously reduces myocardial cell death in the area of partial ischemia, the AAR, limiting the myocardial necrosis to the area completely cut off from blood supply. The antiapoptotic effect of rhEPO observed at 24 h after coronary ligation in our study probably extends beyond the first couple of days after ligation and contributes to subsequent reduction of LV wall remodeling in response to the increased wall stress by precluding the total cell loss from reaching the critical level required for late MI expansion and LV dilatation.
The final infarct size at 8 weeks after coronary ligation is an integral of several processes. The 50% reduction of the apoptosis coupled to any effects of EPO to enhance collateral flow during the first 24 h likely reduces expansion of the early infarct. Although we do not know whether EPO treatment at the time of coronary ligation directly affects later LV remodeling, its effect to reduce the MI size at the early stages is likely a major factor in markedly reducing later LV dilatation. This LV dilatation is associated with an increase in wall stress that, ipso facto, is a stimulus for additional late apoptosis (26), not sampled in the present study. Thus, the reduction of final size in infarct (at 8 weeks) by EPO may be attributable both to a direct reduction of early apoptosis and to a later, indirect reduction of apoptosis due to stretch resulting from LV dilatation, which was less in the EPO-treated group. In other words, it might be assumed that after the first 24 hr a further expansion of the infarct proceeded at a different rate in untreated, ligated and EPO-treated animals. Thus, it is not surprising that final MI reduction by EPO exceeded the reduction of apoptosis measured at the very early stage.
The signaling pathways involved in the antiapoptotic effects of rhEPO are complex (27), and the complete analysis is beyond the scope of this article. The effect of rhEPO on cell survival might involve an interplay of growth factors and cytokines, e.g., transforming growth factor (TGF), tumor necrosis factor, and IL-6. It has been shown that a single dose of rhEPO reduces the serum level of TGF-β1 in patients subjected to dialysis (28), and, in cell culture, rhEPO prevents the apoptosis induced by activin A (a member of the TGF-β superfamily) (29). Although the present results did not include the measure of their cytokines, mediation of the cardioprotective effect of rhEPO through attenuation of the activity of these known promoters of apoptosis might explain an effect of EPO within the myocardium in the apparent absence of EPO-Rs in adult hearts.
If the present results in the rodent MI model are applicable to humans, then a single dose of rhEPO administered around the time of infarct might offer a novel therapeutic opportunity to limit myocardial damage and developing contractile dysfunction. Whereas the single dose of rhEPO in the present study (3,000 units/kg) exceeded the usually recommended repeated doses for the treatment of anemia (150–300 units/kg) and is roughly twice the maximum effective single dose in humans, epoetin alpha is effective in a dose-dependent fashion up to 1,800 units/kg. There are no further increases in the reticulocyte response at 2,400 units/kg and no adverse effects at either of these doses (18). No antibodies to rhEPO had been detected after a single injection of 1,000 units/kg (30). The LD50 for rhEPO has never been reported.
In summary, this paper provides proof of the concept that a single systemic injection of rhEPO after the induction of permanent myocardial ischemia blocks apoptosis, reduces the extent of resulting MI, and attenuates the accompanying LV functional decline. Further studies of the therapeutic window of rhEPO application after MI, of the effect of repeated doses, and of the most clinically relevant effect of rhEPO treatment combined with revascularization will define the scope of possible clinical applications.
Note. While the present article was in review, a study was published (31) in which it was demonstrated that repeated administration of recombinant human EPO (5,000 units/kg of body weight; i.p. daily for 7 days) in a rat coronary ischemic-reperfusion model reduces cardiomyocyte loss and normalizes diastolic hemodynamic dysfunction within 1 week after reperfusion.
Supplementary Material
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: EPO, erythropoietin; EPO-R, EPO receptor; rhEPO, recombinant human EPO; SH, sham; SHnT, SH not treated; SHT, SH treated with rhEPO; MI, myocardial infarction; MInT, MI not treated; MIT, MI treated with rhEPO; LV, left ventricular; LVEDA, LV end-diastolic area; LVESA, LV end-systolic area; LVEDD, LV end-diastolic dimension; LVESD, LV end-systolic dimension; TUNEL, terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling; AAR, area at risk; M-mode, motion mode; LVFS, LV fractional shortening; LVFAC, LV fractional area change.
References
- 1.Youssoufian, H., Longmore, G., Neumann, D., Yoshimura, A. & Lodish, H. F. (1993) Blood 81, 2223–2236. [PubMed] [Google Scholar]
- 2.Juul, S. E. (2000) Clin. Perinatol. 27, 527–541. [DOI] [PubMed] [Google Scholar]
- 3.Liu, Z. Y., Chin, K. & Noguchi, C. T. (1994) Dev. Biol. 166, 159–169. [DOI] [PubMed] [Google Scholar]
- 4.Brines, M. L., Ghezzi, P., Keenan, S., Agnello, D., de Lanerolle, N., Cerami, C., Itri, L. M. & Cerami, A. (2000) Proc. Natl. Acad. Sci. USA 97, 10526–10531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Siren, A. L., Knerlich, F., Poser, W., Gleiter, C. H., Bruck, W. & Ehrenreich, H. (2001) Acta Neuropathol. 101, 271–276. [DOI] [PubMed] [Google Scholar]
- 6.Grimm, C., Wenzel, A., Groszer, M., Mayser, H., Seeliger, M., Samardzija, M., Bauer, C., Gassmann, M. & Reme, C. E. (2002) Nat. Med. 8, 718–724. [DOI] [PubMed] [Google Scholar]
- 7.Yu, X., Shacka, J. J., Eells, J. B., Suarez-Quian, C., Przygodzki, R. M., Beleslin-Cokie, B., Lin, C. S., Nikodem V. M., Hempstead, B., et al. (2002) Development 129, 505–516. [DOI] [PubMed] [Google Scholar]
- 8.Jelkmann, W. (1994) Clin. Invest. 72, S3–S10. [PubMed] [Google Scholar]
- 9.Vairano, M., Russo, C. D., Pozzoli, G., Battaglia, A., Scambia, G., Tringali, G., Aloe-Spiriti, M. A., Preziosi, P. & Navarra, P. (2002) Eur. J. Neurosci. 16, 584–592. [DOI] [PubMed] [Google Scholar]
- 10.Siren, A.-L., Fratelli, M., Brines, M., Goemans, C., Casagrande, S., Lewczuk, P., Keenan, S., Gleiter, C., Pasquali, C., Capobianco, A., et al. (2001) Proc. Natl. Acad. Sci. USA 98, 4044–4049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pfeffer, M. A., Pfeffer, J. M., Steinberg, C. & Finn, P. (1985) Circulation 72, 406–412. [DOI] [PubMed] [Google Scholar]
- 12.Egrie, J. C., Browne, J., Lai, P. & Lin, F. K. (1985) Prog. Clin. Biol. Res. 191, 339–350. [PubMed] [Google Scholar]
- 13.Egrie, J. C., Strickland, T. W., Lane, J., Aoki, K., Cohen, A. M., Smalling, R., Trail, G., Lin, F. K., Browne, J. K. & Hines, D. K. (1986) Immunobiology 172, 213–224. [DOI] [PubMed] [Google Scholar]
- 14.Francis, J., Weiss, R. M., Wei, S. G., Johnson, A. K. & Felder, R. B. (2001) Am. J. Physiol. 281, R1734–R1745. [DOI] [PubMed] [Google Scholar]
- 15.Vivaldi, M. T., Kloner, R. A. & Schoen, F. J. (1985) Am. J. Pathol. 121, 522–530. [PMC free article] [PubMed] [Google Scholar]
- 16.Bialik, S., Geenen, D. L., Sasson, I. E., Cheng, R., Horner, J. W., Evans, S. M., Lord, E. M., Koch, C. J. & Kitsis, R. N. (1997) J. Clin. Invest. 100, 1363–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu, W. C., Rathore, S. S., Wang, Y., Radford M. J. & Krumholz, H. M. (2001) N. Engl. J. Med. 345, 1230–1236. [DOI] [PubMed] [Google Scholar]
- 18.Cheung, W. K., Goon, B. L., Guilfoile, M. C. & Wacholtz M. C. (1998) Clin. Pharmacol. Ther. 64, 412–423. [DOI] [PubMed] [Google Scholar]
- 19.Elder, H., Rosslenbroich, B. & Failing, K. (1989) Blut 59, 184–187. [DOI] [PubMed] [Google Scholar]
- 20.Jaquet, K., Krause, K., Tawakol-Khodai, M., Geidel, S. & Kuck, K. (2002) Microvasc. Res. 64, 326–333. [DOI] [PubMed] [Google Scholar]
- 21.Squadrito, F., Altavilla, D., Squadrito, G., Campo, G. M., Arlotta, M., Quartarone, C., Saitta, A. & Caputi, A. P. (1999) Br. J. Pharmacol. 127, 482–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hughes, S. (2002) J. Pathol. 197, 468–478. [DOI] [PubMed] [Google Scholar]
- 23.Shingo, T., Sorokan, S. T., Shimazaki, T. & Weiss, S. (2001) J. Neurosci. 21, 9733–9743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heeschen, C., Aicher, A., Lehmann, R., Fishtlscherer, S., Vasa, M., Urbich, C., Mildner-Rihm, C., Martin, H., Zeiher, A. M. & Dimmeler S. (2003) Blood 102, 1340–1346. [DOI] [PubMed] [Google Scholar]
- 25.Celik, M., Gökmen, N., Erbayraktar, S., Akhisaroglu, M., Konakc, S., Ulukus, C., Genc, S., Genc, K., Sagiroglu, E., Cerami, A. & Brines, M. (2002) Proc. Natl. Acad. Sci. USA 99, 2258–2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wencker, D., Chandra, M., Nguyen, K., Miao, W., Garantziotis, S., Factor, S. M., Shirani, J., Armstrong, R. C. & Kitsis, R. N. (2003) J. Clin. Invest. 111, 1497–1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sawyer, S. T. & Jacobs-Helber, S. M. (2000) J. Hematother. Stem Cell Res. 9, 21–29. [DOI] [PubMed] [Google Scholar]
- 28.Logofetov, A., Todorov, V., Yotova, P., Zlatarska, S. & Nyagolov, Y. (1998) Arch. Physiol. Biochem. 106, 265–268. [DOI] [PubMed] [Google Scholar]
- 29.Fukuchi, Y., Kizaki, M., Yamato, K., Kawamura, C., Umezawa, A., Hata, J., Nishihara, T. & Ikeda, Y. (2001) Oncogene 20, 704–713. [DOI] [PubMed] [Google Scholar]
- 30.Flaharty, K. K., Caro, J., Erslev, A., Whalen, J. J., Morris, E. M., Bjornsson, T. D. & Vlasses, P. H. (1990) Clin. Pharmacol. Ther. 47, 557–564. [DOI] [PubMed] [Google Scholar]
- 31.Calvillo, L., Latini, R., Kajstura, J., Leri, A., Anversa, P., Ghezzi, P., Salio, M., Cerami, A. & Brines, M. (2003) Proc. Natl. Acad. Sci. USA 100, 4802–4806. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.