Abstract
Ectodomain shedding of epidermal growth factor receptor (EGFR) ligands [e.g., transforming growth factor type α (TGF-α)] and EGFR phosphorylation are implicated in mucin production in airway epithelial cells. Tumor necrosis factor α-converting enzyme (TACE) is reported to cleave precursor of TGF-α, with release of soluble mature TGF-α in various epithelial tissues. We hypothesized that TACE increases the shedding of TGF-α, resulting in EGFR phosphorylation and inducing mucin production in human airway epithelial (NCI-H292) cells. To examine this hypothesis, we stimulated NCI-H292 cells with phorbol 12-myristate 13-acetate (PMA, an activator of TACE) and pathophysiologic stimuli [lipopolysaccharide (LPS) and supernatant from the Gram-negative bacterium Pseudomonas aeruginosa (PA sup)]. PMA, PA sup, and LPS increased MUC5AC gene expression and mucin protein production, effects that were prevented by pretreatment with AG1478, a selective inhibitor of EGFR phosphorylation and by preincubation with an EGFR-neutralizing Ab or with a TGF-α-neutralizing Ab, implicating ligand (TGF-α)-dependent EGFR phosphorylation in mucin production. These stimuli induced release of soluble TGF-α, EGFR phosphorylation, and MUC5AC expression, which were blocked by the metalloprotease inhibitors tumor necrosis factor-α protease inhibitor-1 and tissue inhibitor of metalloprotease-3. We specifically knocked down the expression of metalloprotease TACE by using small interfering RNA for TACE. Knockdown of TACE inhibited PMA-, PA sup-, and LPS-induced TGF-α shedding, EGFR phosphorylation, and mucin production. From these results, we conclude that TACE plays a critical role in mucin production by airway epithelial cells by means of a TACE ligand–EGFR cascade in response to various stimuli.
Mucus hypersecretion is an important feature of chronic inflammatory airway diseases (1) and contributes to their morbidity and mortality. MUC5AC mucin is a major component of mucus in airway epithelial cells (2) and is regulated by means of an epidermal growth factor receptor (EGFR)-signaling pathway (3); ligand-dependent EGFR phosphorylation induces MUC5AC mucin.
Airway epithelial cells produce EGFR and its ligands (4, 5). Transforming growth factor type α (TGF-α) plays a critical role in EGFR phosphorylation, leading to MUC5AC production in airways. TGF-α is synthesized as transmembrane pro-TGF-α, which is processed and released (ectodomain shedding) from the cell surface by metalloproteases (6, 7). Tumor necrosis factor α-converting enzyme (TACE) has been reported to cleave pro-TGF-α into mature soluble TGF-α in various epithelial tissues (8).
TACE is a member of “a disintegrin and metalloprotease” (ADAM) family, a group of zinc-dependent transmembrane metalloproteases (9, 10). TACE is produced in a latent form (11, 12) and is activated by agents such as phorbol 12-myristate 13-acetate (PMA) (13) and reactive oxygen species (12, 14), resulting in substrate cleavage. We hypothesized that TACE activation induces MUC5AC mucin production. The hypothesis is that airway epithelial cells can cleave EGFR pro-ligand on their surfaces into mature soluble ligand, which can then bind to and activate EGFR, resulting in mucin production. We used two stimuli to induce mucin production. First, we studied the effect of PMA because PMA is known to activate TACE (13), but it is not known whether it induces mucin expression. Second, we studied the effect of Pseudomonas aeruginosa (PA), one of the most common pathogens in hypersecretory diseases. PA is known to stimulate mucin production by means of EGFR phosphorylation (15), but the mechanism by which PA causes EGFR phosphorylation is unknown.
First, we examined whether activation of TACE by PMA causes MUC5AC expression in airway epithelial (NCI-H292) cells and, if so, whether increased shedding of soluble TGF-α and EGFR phosphorylation are involved in this process. Next, we examined whether inhibition of TACE activity by application of metalloprotease inhibitors and more importantly by specific knockdown of TACE by using small interfering RNA (siRNA) to silence TACE expression could block PMA-induced TGF-α release, EGFR phosphorylation, and MUC5AC expression. Finally, we examined whether TACE is also involved in PA- and lipopolysaccharide (LPS)-induced TGF-α release, EGFR phosphorylation, and mucin production. Here we show that activation of TACE by these stimuli causes ligand (TGF-α)-dependent EGFR phosphorylation and up-regulates MUC5AC expression. Inhibition of TACE or knockdown of TACE prevented ligand (TGF-α)-dependent EGFR phosphorylation and MUC5AC expression.
Materials and Methods
Materials. PMA was obtained from Sigma. AG1478, calphostin C (CC), Bisindolylmaleimide I, tissue inhibitor of metalloprotease 3 (TIMP-3), tumor necrosis factor α proteinase inhibitor-1 (TAPI-1), EGFR-neutralizing Ab (Ab 3), EGF-neutralizing Ab, and TGF-α-neutralizing Ab were purchased from Calbiochem. Anti-human TACE Ab, anti-phosphotyrosine (PY99) Ab, and anti-human EGFR Ab were obtained from Santa Cruz Biotechnology.
Cell Culture. NCI-H292 cells, a human pulmonary mucoepidermoid carcinoma cell line, were plated at 5–6 × 105 cells in 2 ml in each well of a 6-well plate or at 1–2 × 105 cells in 1 ml in each well of a 24-well plate (both 6- and 24-well plates were purchased from BD Biosciences) and were grown in RPMI medium 1640 containing 10% FCS, penicillin (100 units/ml), streptomycin (100 μg/ml), and Hepes (25 mM) at 37°C in a humidified, 5% CO2/95% air, water-jacketed incubator. After the cells reached confluence, they were serum-starved for 24 h to maintain low basal levels of MUC5AC expression.
Treatment of Cells with PMA, PA Supernatant (PA Sup), or LPS. After 24 h of serum starvation, cells were treated with stimuli as indicated in each experiment. For inhibitor studies, serum-starved cells were pretreated with inhibitors for 30 min before exposure to stimuli. In studies of PMA, cells were treated with PMA (10 ng/ml) for 1 h. Then the cells were washed three times with serum-free medium and cultured for another 24 h in serum-free medium with the same concentrations of inhibitors as in the pretreatment period. In studies of PA sup, PA strain PAO1 was grown in M9 medium, and a cell-free culture supernatant (PA sup) was obtained (15, 16). Cells were incubated with PA sup (1:8 dilution in medium containing 10% FCS) or LPS (from PA; 10 μg/ml in medium containing 10% FCS) for 24 h with or without inhibitors. After 24 h, cell culture supernatants and cell lysates were collected to measure MUC5AC expression.
MUC5AC ELISA and Immunocytochemical Staining. Production of MUC5AC protein in cell lysates and in cell culture supernatants was measured. The amount of MUC5AC protein in each portion was normalized to total protein in cell lysate and was expressed as μg/mg of cell protein. Cells were grown in 24-well plates, and MUC5AC protein was measured by ELISA (17). For MUC5AC immunocytochemical staining, cells were grown on eight-well chamber slides and exposed to stimuli for 24 h. At the end of experiments, cells were fixed and stained (18).
Analysis of Effect of PMA, PA Sup, and LPS on Cleavage and Release of Soluble TGF-α. Cells were grown in six-well plates. After reaching confluence and being serum starved for 24 h, cells were stimulated with PMA (10 ng/ml), PA sup (1:8 dilution), or LPS (10 μg/ml), or maintained in serum-free medium alone or in medium containing 10% FCS alone for 2 h. For inhibitor studies, cells were pretreated with the inhibitors for 30 min before each stimulus was added. To prevent soluble TGF-α from binding to EGFR, an EGFR-neutralizing Ab (Ab-3, 4 μg/ml) was added 30 min before each stimulus was added. Cell supernatants were collected, and TGF-α was measured by using the TGF-α ELISA kit (Oncogene Science) according to the manufacturer's instructions.
RNA Isolation and RT-PCR. Total RNA was isolated by using RNAqueous-4PCR kit (Ambion, Austin, TX), and 2 μg was primed with oligo(dT) and reverse transcribed by using a RETROscript kit (Ambion) in a final volume of 20 μl (RT reaction) according to the manufacturer's instructions. Two microliters of the RT reaction was PCR amplified in a 50-μl reaction by using SuperTaq DNA polymerase (Ambion). Primers for MUC5AC were (forward) 5′-TCCGGCCTCATCTTCTCC-3′ and (reverse) 5′-ACTTGGGCACTGGTGCTG-3′. The expected fragment amplified by PCR was 679 bp. As quantitative controls, primers for Rig/S15 rRNA, which encodes a small ribosomal subunit protein, a housekeeping gene that was constitutively expressed (19), were used. They were 5′-TTCCGCAAGTTCACCTACC-3′ (forward) and 5′-CGGGCCGGCCATGCTTTACG-3′ (reverse). The PCR mixture was denatured at 94°C for 5 min, followed by 30 cycles at 94°C for 30 s, 57°C for 45 s, and 72°C for 45 s. After PCR, 10-μl aliquots were subjected to 1.5% agarose gel electrophoresis and stained with ethidium bromide.
Cell Lysis, Immunoprecipitation, and Immunoblotting. After various treatments, cells were lysed on ice in PBS lysis buffer containing 1% Triton X-100, 1% deoxycholic acid, 50 mM NaF, 1 mM sodium orthovanadate, and proteinase inhibitors (Complete Mini, Roche). Lysates were precleared by centrifugation at 14,000 rpm in a Denville Micro 260D microcentrifuge (Denville Scientific, Metuchen, NJ) for 15 min at 4°C. Protein concentration was determined by using the bicinchoninic acid-based protein assay kit (Pierce). For immunoprecipitation, aliquots of cell lysates containing equal amounts of protein were immunoprecipitated with 4 μg of anti-EGFR Ab and 40 μl of protein A/G-agarose beads (Santa Cruz Biotechnology) for 16 h at 4°C. Precipitates were washed three times with 0.5 ml of PBS, suspended in SDS sample buffer, boiled for 5 min, and subjected to electrophoresis on an SDS/7.5% polyacrylamide gel. For Western blotting, 30 μg of protein from each treatment was subjected to electrophoresis on an SDS/7.5% polyacrylamide gel and transferred to a poly(vinylidene difluoride) membrane (Bio-Rad), which was blocked with 5% BSA, probed with specific primary Abs, washed with PBS, and then probed with secondary Abs conjugated to horseradish peroxidase. Immunoreactive bands were visualized by chemiluminescence with Western blotting luminol reagent (Santa Cruz Biotechnology).
TACE siRNA Preparation and Transfection. To design TACE-specific siRNA duplexes, we selected sequences of the type AAN19 (N, any nucleotide) from the ORF of TACE (ADAM 17) mRNA (GenBank accession no. NM_003183) to obtain 21-nt sense and antisense strand 5 with a GC content <50%. A selected siRNA sequence was submitted to a blast search against the human genome sequence to ensure that only the TACE gene of the human genome was targeted. The selected 21-nt sequences of TACE are (sense) 5′-AAGCTTGATTCTTTGCTCTCA-3′ and (antisense) 5′-AATGAGAGCAAAGAATCAAGC-3′. As a nonspecific siRNA control, a sequence targeting firefly (Photinus pyralis) luciferase (luc) gene (X65324) 153–175 was used (20, 21). The 21-nt double-stranded RNAs were prepared in vitro by Silencer siRNA construction kit (Ambion). siRNA transfection into NCI-H292 cells was carried out by using the Silencer siRNA transfection kit (Ambion). Western blotting was used to detect TACE silencing by siRNA at 48 h after transfection.
Statistical Analysis. Data are presented as mean ± SD (n = 3). ANOVA was used to determine statistically significant differences (P < 0.01).
Results
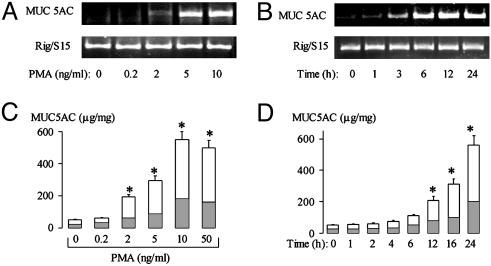
PMA Up-Regulates MUC5AC Expression. PMA increased MUC5AC gene expression in NCI-H292 cells dose-dependently (at 10 ng/ml, PMA increased gene expression 12-fold; Fig. 1A) and time-dependently (with a peak at 6–12 h after PMA stimulation; Fig. 1B). PMA also increased MUC5AC protein production dose-dependently [PMA (10 ng/ml) increased MUC5AC protein 10-fold; Fig. 1C] and time-dependently (a significant increase was seen at 12–24 h after PMA stimulation; Fig. 1D). These findings were confirmed by immunocytochemical staining for MUC5AC (data not shown).
Fig. 1.
Effect of PMA on MUC5AC expression. NCI-H292 cells were treated with vehicle or PMA at various concentrations and times. Total RNA was prepared and subjected to RT-PCR; Rig/S15 was used as an internal marker. (A) Effect of increasing concentrations of PMA given for 6 h on RT-PCR-amplified MUC5AC mRNA expression. (B) Effect of PMA (10 ng/ml) given for various times on RT-PCR-amplified MUC5AC mRNA expression. (C) Effect of increasing concentrations of PMA on total MUC5AC protein production during 24 h by ELISA. (D) Effect of PMA (10 ng/ml) given for various times on total MUC5AC protein production by ELISA. Mucins were measured in the cell lysate (shaded areas) and in the supernatant (unshaded areas). Data in C and D are expressed as mean ± SD. *, P < 0.01, compared with vehicle alone.
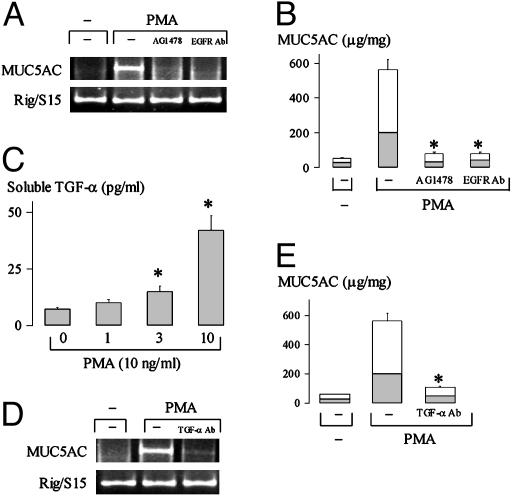
EGFR Activation and EGFR Ligand TGF-α Mediate MUC5AC Expression by PMA. Pretreatment of cells with AG1478, a selective inhibitor of EGFR phosphorylation (22), or with an EGFR-neutralizing Ab, which blocks ligand binding sites on EGFR and inhibits subsequent EGFR phosphorylation, prevented MUC5AC gene expression (Fig. 2A) and protein production (Fig. 2B) by PMA (10 ng/ml), implicating ligand-dependent EGFR phosphorylation in PMA-induced mucin expression. PMA increased release of soluble TGF-α dose-dependently [PMA (10 ng/ml) increased the release 8-fold; Fig. 2C]. The increases of MUC5AC gene expression and protein production by PMA were inhibited by pretreatment of cells with a TGF-α-neutralizing Ab (Fig. 2 D and E) but not by pretreatment with an EGF-neutralizing Ab (data not shown), implicating TGF-α in PMA-induced mucin production. Findings of protein production were confirmed by immunocytochemistry (data not shown).
Fig. 2.
Ligand (TGF-α)-dependent EGFR activation mediates MUC5AC expression by PMA. (A and B) Cells were treated with vehicle alone, PMA alone, PMA after 30-min pretreatment with AG1478 (10 μM), or PMA plus an EGFR-neutralizing Ab (4 ng/ml) for 6 h (A) or 24 h (B). MUC5AC mRNA expression was examined by RT-PCR (A), and mucin production was measured by ELISA (B). *, P < 0.01, compared with PMA alone. (C) Cells were pretreated with an anti-EGFR-neutralizing Ab for 30 min to block EGFR-ligand-binding sites, and then the cells were stimulated with vehicle or PMA at different concentrations for 2 h. Soluble TGF-α in the supernatant was measured by ELISA. *, P < 0.01, compared with vehicle alone. (D) Cells were treated with vehicle alone, PMA alone, or PMA plus a TGF-α-neutralizing Ab (4 ng/ml) for 6 h. MUC5AC mRNA expression was examined by RT-PCR. Results in three separate experiments were similar. (E) Cells were treated with vehicle alone (left), PMA alone (center), or PMA plus a TGF-α-neutralizing Ab (4 ng/ml) (right) for 24 h in the absence or presence of a TGF-α-neutralizing Ab. MUC5AC mucin protein production was examined by ELISA. Mucins were measured in the cell lysate (shaded areas) and in the supernatant (unshaded areas). Data in B, C, and E are expressed as mean ± SD. *, P < 0.01, compared with PMA alone.
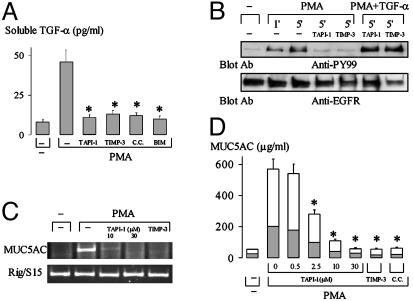
Inhibition of Metalloprotease Activation Prevents PMA-Induced TGF-α Shedding, EGFR Phosphorylation, and MUC5AC Expression. PMA-induced release of soluble TGF-α was inhibited by two metalloprotease inhibitors, TAPI-1 (12) and TIMP-3 (23, 24) (Fig. 3A), but not by cysteine protease inhibitor (leupeptin, 50 μM; data not shown) or serine protease inhibitor (aprotinin, 10 μg/ml; data not shown), implicating metalloprotease activation in PMA-induced release of soluble TGF-α. PKC inhibitors CC and Bisindolylmaleimide I also inhibited PMA-induced shedding of soluble TGF-α, indicating the involvement of PKC in metalloprotease activation (Fig. 3A).
Fig. 3.
Effect of inhibitors of metalloprotease and PKC on TGF-α shedding, EGFR phosphorylation, and mucin expression. (A) Cells were pretreated with an anti-EGFR neutralizing Ab for 30 min to block EGFR-ligand-binding sites and then with TAPI-1 (30 μM) or TIMP-3 (10 μg/ml), or with PKC inhibitors [CC and Bisindolylmaleimide I (200 nM), respectively] for 30 min, and then stimulated with PMA (10 ng/ml) for 2 h. Supernatants were collected for measurement of soluble TGF-α by ELISA. *, P < 0.01, compared with PMA alone. (B) Cells were cultured to confluence. After pretreatment with or without inhibitors as shown [TAPI-1 (30 μM), TIMP-3 (10 μg/ml)], cells were stimulated with PMA (10 ng/ml) for various times or with PMA (10 ng/ml) and TGF-α (20 ng/ml) for 5 min. After lysis, EGFR was immunoprecipitated with anti-EGFR mAb and then immunoblotted with anti-phosphotyrosine Ab (PY99, Upper) and reprobed with polyclonal anti-EGFR Ab (Lower). (C) Cells were pretreated with TAPI-1 (10 or 30 μM) or TIMP-3 (10 μg/ml) for 30 min and then treated with PMA (10 ng/ml) for 6 h. MUC5AC gene expression was measured by RT-PCR. (D) Cells were pretreated with inhibitors for 30 min and then stimulated with PMA (10 ng/ml) for 24 h in the presence of the respective inhibitors. MUC5AC mucin production in the cell lysate (shaded areas) and in the supernatant (unshaded areas) was analyzed by ELISA. *, P < 0.01, compared with PMA alone.
Pretreatment with TAPI-1 or TIMP-3 inhibited PMA-induced EGFR phosphorylation (Fig. 3B). However, pretreatment with TAPI-1 or TIMP-3 did not inhibit EGFR phosphorylation by PMA when exogenous TGF-α (20 ng/ml) was added (Fig. 3B). These results implicate metalloprotease activation and TGF-α release in PMA-induced EGFR phosphorylation.
Pretreatment of cells with TAPI-1 or TIMP-3 inhibited PMA-induced MUC5AC gene expression (Fig. 3C) and protein production (Fig. 3D). A PKC inhibitor (CC) had similar inhibitory effects on mucin production (Fig. 3D). Immunocytochemistry confirmed these results (data not shown).
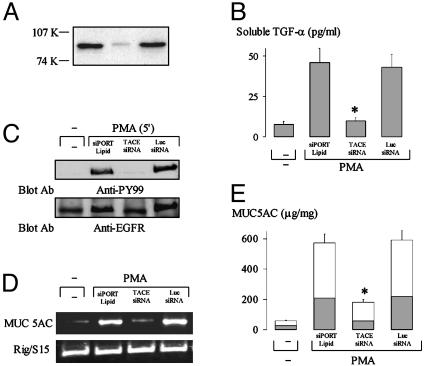
Metalloprotease TACE Mediates PMA-Induced EGFR Phosphorylation and MUC5AC Expression. Metalloprotease TACE is reported to cleave pro-TGF-α (8). To examine whether TACE is involved in PMA-induced TGF-α release, EGFR phosphorylation, and mucin expression, we knocked down TACE expression ≈90% in NCI-H292 cells by transfection of TACE siRNA (Fig. 4A). TACE siRNA inhibited PMA-induced TGF-α shedding (Fig. 4B), EGFR phosphorylation (Fig. 4C), MUC5AC mRNA expression (Fig. 4D), and mucin protein production evaluated by ELISA (Fig. 4E). luc siRNA (nonspecific control) was without effect (Fig. 4). These results strongly implicate TACE in TGF-α shedding, EGFR phosphorylation, and up-regulation of MUC5AC expression in human airway epithelial cells.
Fig. 4.
Effect of knockdown of TACE on PMA-induced TGF-α release, EGFR phosphorylation, and MUC5AC expression. (A) Western blot of NCI-H292 cells treated with siPORT Lipid (transfection agent for siRNA; Ambion) alone (left) or transfected with TACE siRNA (10 nM, center) or luc siRNA (10 nM, right, as negative control) by using anti-TACE mAb. (B) Cells were treated with siPORT Lipid, or transfected with TACE siRNA (10 nM) or luc siRNA (10 nM). Later (48 h), cells were stimulated with PMA (10 ng/ml) for 2 h as described for Fig. 2C. Soluble TGF-α in the supernatant was measured by ELISA. *, P < 0.01, compared with cells treated with siPORT Lipid and transfected with luc siRNA. (C–E) Cells were treated with siPORT Lipid or transfected with TACE siRNA or luc siRNA. Forty-eight hours later, cells were treated with vehicle or PMA (10 ng/ml) for 5 min to examine the effect of knockdown of TACE on EGFR phosphorylation (C) (as described for Fig. 3B), or treated with vehicle or PMA (10 ng/ml) for 6 h to examine MUC5AC mRNA expression (D) or for 24 h to measure mucin protein production by ELISA (E). MUC5AC mucin production in the cell lysate (shaded areas) and in the supernatant (unshaded areas) was analyzed by ELISA. Data in B and E are expressed as mean ± SD. *, P < 0.01, compared with corresponding controls.
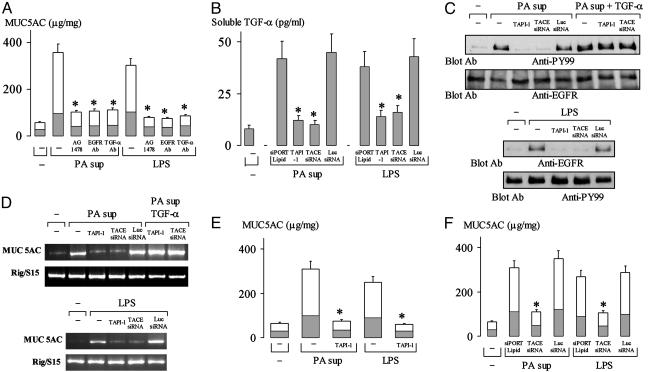
TACE Mediates PA- and LPS-Induced MUC5AC Expression by Means of Ligand (TGF-α)-Dependent EGFR Phosphorylation. MUC5AC protein production by PA sup and by LPS was inhibited by pretreatment with AG1478, by an EGFR-neutralizing Ab, or by a TGF-α-neutralizing Ab (Fig. 5A), implicating ligand (TGF-α)-dependent EGFR phosphorylation in PA sup- and LPS-induced mucin production. TAPI-1 pretreatment or knockdown of TACE by TACE siRNA inhibited PA sup- and LPS-induced TGF-α release (Fig. 5B), prevented PA sup-induced (Fig. 5C Upper) and LPS-induced (Fig. 5C Lower) EGFR phosphorylation, MUC5AC mRNA expression (Fig. 5D), and mucin protein production (Fig. 5 E and F). Addition of exogenous TGF-α (20 ng/ml) reversed the inhibitory effects of TAPI-1 and TACE siRNA on PA sup-induced EGFR phosphorylation (Fig. 5C, three right lanes in Upper), MUC5AC mRNA expression (Fig. 5D, two right lanes in Upper), and protein production (data not shown). luc siRNA was without effect (Fig. 5 B–F). PKC inhibitors (CC and Bisindolylmaleimide I) also blocked PA sup- and LPS-induced TGF-α release, EGFR phosphorylation, MUC5AC mRNA expression, and mucin production (data not shown).
Fig. 5.
Role of TACE in PA sup- and LPS-induced EGFR phosphorylation and MUC5AC production. (A) Cells were pretreated with or without EGFR inhibitor, AG1478 (10 μM), or an EGFR-neutralizing Ab (4 ng/ml), or a TGF-α-neutralizing Ab (4 ng/ml) for 30 min, and then were treated with PA sup (1:8 dilution) or LPS (10 μg/ml) for 24 h in the presence or absence of the individual inhibitor or Abs. Mucin protein production was examined by ELISA. *, P < 0.01, compared with PA sup alone or LPS alone. (B) Cells were treated with siPORT Lipid or transfected with TACE siRNA (10 nM) or luc siRNA (10 nM) and incubated for 48 h, or cells were pretreated with TAPI-1 (30 μM) for 30 min. Then the cells were stimulated with PMA (10 ng/ml) for 2 h (as described for Fig. 2C). Soluble TGF-α in the supernatant was measured by ELISA. *, P < 0.01, compared with cells treated with siPORT Lipid and transfected with luc siRNA. (C–F) Cells were pretreated with vehicle or siPORT Lipid, or were transfected with TACE siRNA (10 nM) or luc siRNA (10 nM) and incubated for 48 h, or cells were pretreated with TAPI-1 (30 μM) for 30 min. Then the cells were treated with PA sup (1:8 dilution), PA sup (1:8 dilution) plus TGF-α (20 ng/ml), or with LPS (10 μg/ml) for 30 min to examine EGFR phosphorylation (C) (as described for Fig. 3B). The cells were treated with PA sup (1:8 dilution) or with PA sup plus TGF-2 (20 ng/ml) for 10 h to analyze MUC5AC mRNA expression by RT-PCR (D). The cells were treated with PA sup (1:8 dilution) or with LPS (10 μg/ml) for 24 h to measure mucin protein production by ELISA (E and F). Data in A, B, E, and F are expressed as mean ± SD. *, P < 0.01, compared with corresponding controls.
Treatment of cells with the Gram-positive bacterial product (lipoteichoic acid from Staphylococcus aureus, 10–50 μg/ml for 24 h) did not induce significant MUC5AC mRNA expression when examined by RT-PCR or mucin protein production evaluated by ELISA and by immunohistochemistry (data not shown).
Discussion
Here we show that PMA, supernatant from the Gram-negative bacterium PA, and LPS increase MUC5AC expression in cultured human airway epithelial cells by activation of metalloprotease TACE. These data are based on the discovery that PMA-, PA sup-, and LPS-induced mucin production are blocked by metalloprotease inhibitors (TAPI-1 and TIMP-3) and by specific knockdown of TACE expression. Activation of TACE by these stimuli increases the shedding of soluble TGF-α, which binds to and activates EGFR, resulting in increased MUC5AC expression.
EGFR phosphorylation is recognized as a critical event in MUC5AC mucin production in response to stimuli (e.g., PA sup, LPS, and neutrophil elastase) in human airway epithelial cells (15, 25). However, the mechanism by which these stimuli cause EGFR phosphorylation was unknown. To investigate this mechanism, we used PMA, a widely used stimulus known to increase ectodomain shedding of various cell surface molecules, [e.g., TNF-α, TGF-α (26), p75TNF receptor (12), L-selectin (12), and β-amyloid precursor protein (27)] and to cause EGFR phosphorylation. We show that PMA increases MUC5AC expression dose- and time-dependently (Fig. 1).
To investigate the role EGFR phosphorylation in PMA-induced mucin expression, we pretreated cells with AG1478 before PMA treatment to block EGFR phosphorylation or with an EGFR-neutralizing Ab before PMA treatment to block EGFR-ligand-binding sites on the cell surface. These pretreatments inhibited the mucin production by PMA (Fig. 2 A and B), implicating ligand-dependent EGFR phosphorylation in PMA-induced mucin production. We further show that PMA causes rapid shedding of soluble TGF-α (Fig. 2C).
To explore the role of TGF-α in PMA-induced MUC5AC expression, we pretreated the cells with a TGF-α-neutralizing Ab, which inhibited mucin production by PMA (Fig. 2 D and E), implicating TGF-α shedding in PMA-induced MUC5AC mucin production. Although it is possible that other EGFR ligands (e.g., EGF, heparin-binding EGF-like growth factor, and amphiregulin) could also be involved in PMA-induced mucin production, the fact that a TGF-α-neutralizing Ab prevented ≈90% of the effect (Fig. 2 D and E) suggests that TGF-α plays a major role in the response.
TGF-α is synthesized as a 20- to 22-kDa transmembrane precursor, pro-TGF-α (28–30). Newly synthesized pro-TGF-α is cleaved twice by metalloproteases at the Ala-39–Val-40 and Ala-89–Val-90 sequences, releasing 6-kDa soluble TGF-α (6). TACE, a member of the ADAM family proteases, which belongs to the metzincin superfamily, has recently been reported to be responsible for the ectodomain shedding of TGF-α in various epithelial tissues (8, 12). To examine a possible role for metalloprotease TACE in ectodomain shedding of TGF-α in airway epithelial cells, we used metalloprotease inhibitors [TAPI-1 (12, 31) and TIMP-3 (23, 24)]. Both TAPI-1 and TIMP-3 inhibit ectodomain shedding of TNF-α, p55 TNF-α receptor, and L-selectin, which are known substrates of TACE. TAPI-1 and TIMP-3 blocked TGF-α release (Fig. 3A), EGFR phosphorylation (Fig. 3B), MUC5AC gene expression (Fig. 3C), and protein production (Fig. 3D) by PMA, suggesting a possible role of TACE in PMA-induced MUC5AC expression. Because inhibitors such as TAPI-1 and TIMP-3 are not highly selective, we verified the involvement of TACE in PMA-induced TGF-α release, EGFR phosphorylation, and mucin expression by knocking down TACE protein expression specifically by using TACE siRNA (Fig. 4A). siRNA has been reported to be the most specific and potent means for silencing gene expression and protein production (20, 21). Knockdown of TACE expression inhibited PMA-induced TGF-α shedding, EGFR phosphorylation, and mucin expression, indicating that TACE, activated by PMA, is responsible for TGF-α shedding, EGFR phosphorylation, and mucin production by PMA in NCI-H292 cells.
TACE is synthesized in a latent form, which contains an inhibitory prodomain, a catalytic metalloprotease domain, a disintegrin domain, a cysteine-rich domain, a transmembrane domain, and a cytoplasmic domain (9, 32, 33). A thiol group from a cysteine residue in the prodomain interacts with zinc in the catalytic domain and thereby inactivates TACE (11, 12). Disruption of this cysteine-zinc bond results in a conformational change and the generation of activated TACE (11, 12). Among the proposed mechanisms of TACE activation are the following:
(i) PKC activation by stimuli (e.g., PMA) causes a conformational change in the extracellular domain of TACE, thereby exposing its catalytic domain. However, the mechanism of TACE activation by PKC is unclear. It is suggested that PKC causes serine phosphorylation in the cytoplasmic domain of TACE, leading to a conformational change, which breaks the cysteine-zinc bond, unmasking the catalytic site, which binds to and cleaves substrates of TACE. Our results showing that PKC inhibitors inhibit PMA-induced TGF-α shedding (Fig. 3A) and MUC5AC protein production (Fig. 3D) support the involvement of a PKC-signaling pathway in TACE activation by PMA.
(ii) Cysteine oxidation in the prodomain unmasks the catalytic site of TACE. It has been reported that reactive oxygen species or nitric oxide can attack the cysteine sulfhydryl moiety in the prodomain of TACE and release it from coordination with the catalytic zinc, thereby activating latent TACE (12, 14). The fact that H2O2 mimics PMA in increasing the shedding of TACE substrates supports this explanation (12). Our observation that reactive oxygen species scavengers inhibit PMA-induced mucin production partially (data not shown) also supports this oxidation theory. Indeed, the involvement of a PKC-signaling pathway and the oxidation theory are not contradictory because PKC activation has been reported to generate reactive oxygen species (34, 35).
Having elucidated the mechanism that PMA induces MUC5AC expression by means of TACE activation, which leads to TGF-α release and EGFR phosphorylation, we examined whether this mechanism could also be extended to pathophysiological stimuli that induce mucin production. PA infection in airways is a major cause of mucin overproduction. PA sup and its major component, LPS, have been reported to cause MUC5AC expression in airway epithelial cells by means of EGFR phosphorylation (15, 16). However, the mechanism by which PA sup and LPS cause EGFR activation was previously unknown. Here we show that PA sup and LPS increase MUC5AC mucin production by means of ligand (TGF-α)-dependent EGFR phosphorylation (Fig. 5A). Then we show that metalloprotease TACE mediates soluble TGF-α release (Fig. 5B) and TGF-α-dependent EGFR phosphorylation by PA sup and by LPS (Fig. 5C). Finally, we show that TACE is responsible for PA sup- and LPS-induced MUC5AC gene expression and mucin production in airway epithelial (NCI-H292) cells (Fig. 5 D–F). These results were from studies performed in a cancer cell line (NCI-H292 cells), which may express different phenotypes from those expressed in primary cultures of airway epithelia. Even primary cell cultures may differ from the in vivo situation.
The Gram-positive bacterium Staphylococcus aureus and its product, lipoteichoic acid, have been reported to increase MUC2 gene transcription (36) in colon tumor cell line (HM3 cells) by an ADAM 10-dependent EGFR phosphorylation. However, lipoteichoic acid from S. aureus (10–50 μg/ml for 24 h) did not cause a significant MUC5AC protein production in NCI-H292 cells under the same cell culture conditions as studied for MUC5AC expression by PA sup or LPS.
In summary, we show that stimulation of NCI-H292 cells with PMA, PA sup, and LPS results in TACE activation, which cleaves pro-TGF-α into soluble mature TGF-α that is free to bind to EGFR. The subsequent ligand-dependent EGFR phosphorylation results in mucin production in airway epithelial cells. These findings are especially important because PA infections are implicated in mucus hypersecretion associated with respiratory diseases. Our results showing that TACE-induced cleavage of EGFR ligand results in EGFR activation and subsequent mucin production suggest novel mechanisms for therapeutic intervention in hypersecretory diseases.
Acknowledgments
This work was supported by private funding.
Abbreviations: EGFR, epidermal growth factor receptor; LPS, lipopolysaccharide; PA, Pseudomonas aeruginosa; PA sup, PA supernatant; PMA, phorbol 12-myristate 13-acetate; siRNA, small interfering RNA; TACE, tumor necrosis factor α-converting enzyme; TAPI-1, tumor necrosis factor α protease inhibitor-1; TGF-α, transforming growth factor type α; TIMP-3, tissue inhibitor of metalloprotease 3; ADAM, a disintegrin and metalloprotease; luc, luciferase; CC, calphostin C.
References
- 1.Nadel, J. A. (2002) in Chronic Obstructive Lung Disease, eds. Voelkel, N. F. & MacNee, W. (BC Decker, Hamilton, ON, Canada), pp. 161–174.
- 2.Rose, M. C. & Gendler, S. J. (1997) in Airway Mucus: Basic Mechanism and Clinical Perspectives, eds. Rogers, D. F. & Lethem, M. L. (Birkhauser, Basel), pp. 41–66.
- 3.Takeyama, K., Jung, B., Shim, J. J., Burgel, P. R., Dao-Pick, T., Ueki, I. F., Protin, U., Kroschel, P. & Nadel, J. A. (2001) Am. J. Physiol. 280, L165–L172. [DOI] [PubMed] [Google Scholar]
- 4.Kommoss, F., Colley, M., Hart, C. E. & Franklin, W. A. (1990) Mol. Cell. Probes 4, 11–23. [DOI] [PubMed] [Google Scholar]
- 5.Amishima, M., Munakata, M., Nasuhara, Y., Sato, A., Takahashi, T., Homma, Y. & Kawakami, Y. (1998) Am. J. Respir. Crit. Care Med. 157, 1907–1912. [DOI] [PubMed] [Google Scholar]
- 6.Hinkle, C. L., Mohan, M. J., Lin, P., Yeung, N., Rasmussen, F., Milla, M. E. & Moss, M. L. (2003) Biochemistry 42, 2127–2136. [DOI] [PubMed] [Google Scholar]
- 7.Sunnarborg, S. W., Hinkle, C. L., Stevenson, M., Russell, W. E., Raska, C. S., Peschon, J. J., Castner, B. J., Gerhart, M. J., Paxton, R. J., Black, R. A., et al. (2002) J. Biol. Chem. 277, 12838–12845. [DOI] [PubMed] [Google Scholar]
- 8.Peschon, J. J., Slack, J. L., Reddy, P., Stocking, K. L., Sunnarborg, S. W., Lee, D. C., Russell, W. E., Castner, B. J., Johnson, R. S., Fitzner, J. N., et al. (1998) Science 282, 1281–1284. [DOI] [PubMed] [Google Scholar]
- 9.Black, R. A., Rauch, C. T., Kozlosky, C. J., Peschon, J. J., Slack, J. L., Wolfson, M. F., Castner, B. J., Stocking, K. L., Reddy, P., Srinivasan, S., et al. (1997) Nature 385, 729–733. [DOI] [PubMed] [Google Scholar]
- 10.Schlondorff, J. & Blobel, C. P. (1999) J. Cell Sci. 112, 3603–3617. [DOI] [PubMed] [Google Scholar]
- 11.Black, R. A. (2002) Int. J. Biochem. Cell Biol. 34, 1–5. [DOI] [PubMed] [Google Scholar]
- 12.Zhang, Z., Oliver, P., Lancaster, J. J., Schwarzenberger, P. O., Joshi, M. S., Cork, J. & Kolls, J. K. (2001) FASEB J. 15, 303–305. [DOI] [PubMed] [Google Scholar]
- 13.Doedens, J. R. & Black, R. A. (2000) J. Biol. Chem. 275, 14598–14607. [DOI] [PubMed] [Google Scholar]
- 14.Zhang, Z., Kolls, J. K., Oliver, P., Good, D., Schwarzenberger, P. O., Joshi, M. S., Ponthier, J. L. & Lancaster, J. R., Jr. (2000) J. Biol. Chem. 275, 15839–15844. [DOI] [PubMed] [Google Scholar]
- 15.Kohri, K., Ueki, I. F., Shim, J. J., Burgel, P. R., Oh, Y. M., Tam, D. C., Dao-Pick, T. & Nadel, J. A. (2002) Eur. Respir. J. 20, 1263–1270. [DOI] [PubMed] [Google Scholar]
- 16.Dohrman, A., Miyata, S., Gallup, M., Li, J. D., Chapelin, C., Coste, A., Escudier, E., Nadel, J. & Basbaum, C. (1998) Biochim. Biophys. Acta 1406, 251–259. [DOI] [PubMed] [Google Scholar]
- 17.Takeyama, K., Dabbagh, K., Lee, H. M., Agusti, C., Lausier, J. A., Ueki, I. F., Grattan, K. M. & Nadel, J. A. (1999) Proc. Natl. Acad. Sci. USA 96, 3081–3086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takeyama, K., Dabbagh, K., Jeong Shim, J., Dao-Pick, T., Ueki, I. F. & Nadel, J. A. (2000) J. Immunol. 164, 1546–1552. [DOI] [PubMed] [Google Scholar]
- 19.Kitagawa, M., Takasawa, S., Kikuchi, N., Itoh, T., Teraoka, H., Yamamoto, H. & Okamoto, H. (1991) FEBS Lett. 283, 210–214. [DOI] [PubMed] [Google Scholar]
- 20.Elbashir, S. M., Harborth, J., Lendeckel, W., Yalcin, A., Weber, K. & Tuschl, T. (2001) Nature 411, 494–498. [DOI] [PubMed] [Google Scholar]
- 21.Harborth, J., Elbashir, S. M., Bechert, K., Tuschl, T. & Weber, K. (2001) J. Cell Sci. 114, 4557–4565. [DOI] [PubMed] [Google Scholar]
- 22.Levitzki, A. & Gazit, A. (1995) Science 267, 1782–1788. [DOI] [PubMed] [Google Scholar]
- 23.Borland, G., Murphy, G. & Ager, A. (1999) J. Biol. Chem. 274, 2810–2815. [DOI] [PubMed] [Google Scholar]
- 24.Amour, A., Slocombe, P. M., Webster, A., Butler, M., Knight, C. G., Smith, B. J., Stephens, P. E., Shelley, C., Hutton, M., Knauper, V., et al. (1998) FEBS Lett. 435, 39–44. [DOI] [PubMed] [Google Scholar]
- 25.Kohri, K., Ueki, I. F. & Nadel, J. A. (2002) Am. J. Physiol. 283, L531–L540. [DOI] [PubMed] [Google Scholar]
- 26.Pandiella, A. & Massagué, J. (1991) Proc. Natl. Acad. Sci. USA 88, 1726–1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Slack, B. E., Ma, L. K. & Seah, C. C. (2001) Biochem. J. 357, 787–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Derynck, R., Roberts, A. B., Winkler, M. E., Chen, E. Y. & Goeddel, D. V. (1984) Cell 38, 287–297. [DOI] [PubMed] [Google Scholar]
- 29.Lee, D. C., Fenton, S. E., Berkowitz, E. A. & Hissong, M. A. (1995) Pharmacol. Rev. 47, 51–85. [PubMed] [Google Scholar]
- 30.Marquardt, H., Hunkapiller, M. W., Hood, L. E. & Todaro, G. J. (1984) Science 223, 1079–1082. [DOI] [PubMed] [Google Scholar]
- 31.Zhao, J., Chen, H., Wang, Y. L. & Warburton, D. (2001) Int. J. Dev. Biol. 45, 623–631. [PubMed] [Google Scholar]
- 32.Milla, M. E., Leesnitzer, M. A., Moss, M. L., Clay, W. C., Carter, H. L., Miller, A. B., Su, J. L., Lambert, M. H., Willard, D. H., Sheeley, D. M., et al. (1999) J. Biol. Chem. 274, 30563–30570. [DOI] [PubMed] [Google Scholar]
- 33.Reddy, P., Slack, J. L., Davis, R., Cerretti, D. P., Kozlosky, C. J., Blanton, R. A., Shows, D., Peschon, J. J. & Black, R. A. (2000) J. Biol. Chem. 275, 14608–14614. [DOI] [PubMed] [Google Scholar]
- 34.Datta, R., Yoshinaga, K., Kaneki, M., Pandey, P. & Kufe, D. (2000) J. Biol. Chem. 275, 41000–41003. [DOI] [PubMed] [Google Scholar]
- 35.Sagone, A. L., Jr., Husney, R., Guter, H. & Clark, L. (1984) J. Immunol. 133, 1488–1494. [PubMed] [Google Scholar]
- 36.Lemjabbar, H. & Basbaum, C. (2002) Nat. Med. 8, 41–46. [DOI] [PubMed] [Google Scholar]