Abstract
Lyme borreliosis, or Lyme disease (LD), is a tick-borne zoonotic infection of biomedical significance, caused by Borrelia burgdorferi sensu lato (s.l.) spirochetes and transmitted by Ixodes species ticks. It usually circulates among wildlife vertebrate reservoirs and vector ticks but may infect humans, causing multisystem problems. In far western and northern North America, the host reservoirs, tick vectors, and genospecies of Borrelia are well known but not so in the southern U.S., where there is controversy as to the presence of “true” LD. Here we report the presence of the LD spirochete B. burgdorferi sensu stricto (s.s.) and Borrelia bissettii, three main reservoir hosts, and two enzootic tick vectors in the southeastern U.S. The two enzootic tick vectors, Ixodes affinis and Ixodes minor, rarely bite humans but are more important than the human biting “bridge” vector, Ixodes scapularis, in maintaining the enzootic spirochete cycle in nature. We also report extraordinary longevities and infections in the reservoir rodents Peromyscus gossypinus, Sigmodon hispidus, and Neotoma floridana.
Lyme borreliosis (LB) is an infection of public health importance with endemic foci in North America and Eurasia. It is the most common vector-borne infectious disease in the U.S. (1) and Europe (2). The disease may affect the skin, joints, and cardiovascular and nervous systems. It may range from severe to mild or even asymptomatic and may become chronic if not treated promptly. The causative agent of Lyme disease (LD) is a spirochete, Borrelia burgdorferi sensu lato (s.l.), which is transmitted primarily by Ixodes spp. ticks (3). LB is a zoonosis in which humans and domestic animals are incidental to its usual wildlife reservoir hosts (4). The primary reservoir hosts in hyperendemic foci of the spirochete in the northeastern and north central U.S. are species of Peromyscus mice (5), although several other mammalian and avian species are reservoir-competent to various degrees and can be important locally throughout the U.S. (6) and globally (7). It is important to identify the main reservoir host species in each particular geographic area, because the vertebrate species composition may affect local B. burgdorferi abundance in nature. It is also important to identify the main local vector tick species responsible for transmission of the spirochete to humans and the tick enzootic vectors, as well as the local genospecies of B. burgdorferi s.l.
At least 11 genospecies comprise the B. burgdorferi s.l. complex worldwide, and three of these occur in the southeastern U.S.: Borrelia andersonii, Borrelia bissettii, and B. burgdorferi sensu stricto (s.s.) (8, 9). Of the 11 genospecies described globally, only three have been confirmed to have been cultured from humans: B. burgdorferi s.s. (North America and western Europe), Borrelia garinii, and Borrelia afzelii (Europe and Asia). Additionally, B. bissettii was reportedly cultured from several patients in Slovenia (10), but this has yet to be confirmed.
In North America, two tick species are known to transmit B. burgdorferi s.s. to humans, Ixodes pacificus in the far west and Ixodes scapularis in the east. In the U.S., >80% of LD cases occur in the northeastern and mid-Atlantic states (1). Although these two tick species transmit B. burgdorferi to humans, they can also serve as enzootic vectors among several species of mammals and birds. In fact, in the northeast and north central states, I. scapularis is also the main enzootic vector. In contrast, in California, Ixodes spinipalpis appears to be more important than I. pacificus in maintaining the enzootic cycle of the spirochete, although it rarely bites humans (11). There is greater spirochete and tick vector diversity in California and the southeastern U.S. than in the northern states (8, 9, 11), which prompted us to hypothesize that an analogous situation regarding enzootic tick vectors in California might exist in the South. We also wanted to determine the main vertebrate reservoirs of B. burgdorferi in this region.
Materials and Methods
Rodent Collections, Spirochete Isolation, and Characterizations. A variety of rodents and ticks were collected from nine sites in Georgia, seven in South Carolina, and five in Florida from 1991 through 1999 and inspected for the presence and prevalence of B. burgdorferi s.l. (8, 9, 12–16). Rodents were live-trapped by using an assortment of traps. Ticks were removed from the rodents, counted, and identified. They were also collected from vegetation via dragging or flagging a 1-m-square white flannel cloth over low vegetation. We used the most conservative method available to determine infection, i.e., isolation of spirochetes in culture medium from tissues of collected vertebrates and ticks. Urinary bladders and/or ear biopsies from the rodents and tick tissues were inoculated into Barbour–Stoenner–Kelly H medium and incubated at 32–34°C. Cultures were subsequently examined for spirochetes by dark-field microscopy for 6 weeks at ×400. Before inoculation of tissues, they were processed as reported (12). Spirochetal isolates were analyzed by using several methods, including indirect immunofluorescence with several mAbs, polyclonal Abs, and Western blotting (17). Isolates were also screened by PCR for five known DNA target sequences specifically found in B. burgdorferi reference strain B-31, and SDS/PAGE protein profiles. Additionally, restriction fragment length polymorphism and sequence analyses of rrf-rrl intergenic spacer amplicon, ospC, flaB, and rrs genes were performed on spirochete isolates (8, 9, 12, 17).
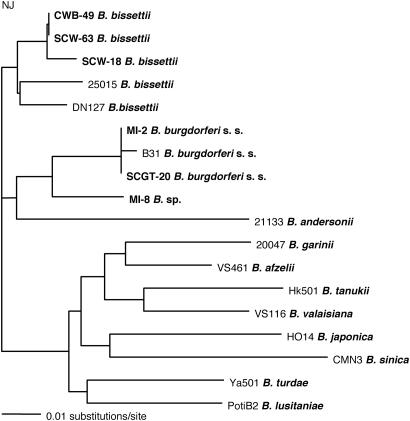
Phylogenetic Tree. The neighbor-joining tree was constructed with paup (Ver. 4.0) software (18) and is based on a comparison of 258-bp nucleotides of the rrf-rrl intergenic spacer sequence. The tree was compared with trees produced by maximum likelihood, parsimony, and unweighted pair group method with arithmetic mean methods with paup software, and the four methods produced similar results.
Results
Reservoir and Tick Collections. Peromyscus gossypinus was the most frequently trapped small mammal during a period of >10 years in our study areas in Georgia, Florida, and South Carolina. Of the 485 mammals collected in Georgia, 236 (48%) were P. gossypinus; in Florida, 74 (42%) of 176; and in South Carolina, 97 (41%) of 237 (13). Sigmodon hispidus was the second most abundant species collected in Georgia (55 of 236, 23%); in Florida (64 of 176, 36%); and in South Carolina (60 of 237, 25%). The third most abundant species of the 485 mammals in Georgia was a virtual tie between the 54 (11%) house mice Mus musculus and the 52 (11%) Neotoma floridana eastern woodrats. In Florida, the third most abundant mammal was the Norway rat, Rattus norvegicus. In South Carolina the third most abundant was N. floridana (21%) (13).
Five species of ticks were commonly recovered from cotton mice, hispid cotton rats, or eastern woodrats during this study: the Gulf Coast tick Amblyomma maculatum, the American dog tick Dermacentor variabilis, Ixodes affinis, Ixodes minor, and the blacklegged tick I. scapularis. Also, a larval Amblyomma dissimile (typically a reptile tick) was collected from one cotton mouse in Florida. Some coinfestations representing different tick species parasitizing the same host at the same time were recorded, especially on N. floridana, and to a lesser extent on P. gossypinus. Most coinfestations involved D. variabilis cofeeding with either I. scapularis, I. minor, or A. maculatum. The number of rodents examined for ticks, infestation prevalence (percent of rodents infested), mean intensities (mean per infested rodent), and numbers and stages of ticks recovered in Georgia, South Carolina, and Florida were recorded. The data on numbers of rodents examined for ticks, infestation prevalances, mean intensities, and numbers and stages of ticks recovered can be found in Appendix A, which is published as supporting information on the PNAS web site, www.pnas.org. There was an increased abundance of immature stages of I. scapularis on rodents in coastal and island habitats.
Prevalences of Borrelia and Identification. The prevalence of naturally occurring spirochete infections in P. gossypinus from five locations in Georgia, four in Florida, and five in South Carolina ranged from 0% to 69% (Table 1). Among S. hispidus, percentages of infectivity varied from 0% to 28% in Georgia, 0% to 13% (except for 100% for the only rat captured at Faver–Dykes) in Florida, and 0% to 80% in South Carolina. Among N. floridana, prevalences ranged from 13% to 35% in Georgia, zero in the one area from which we collected woodrats in Florida, and 18% to 78% in South Carolina. Interestingly, no spirochetes were isolated from a series of other small and medium-size mammals from Georgia (144 animals of 13 species), South Carolina (18 animals of 5 species), or Florida (77 animals of 7 species). The species can be found in Appendix B, which is published as supporting information on the PNAS web site.
Table 1. Prevalence of B. burgdorferi s.l. in three species of rodents, P. gossypinus, S. hispidus, and N. floridana.
| Location | P. gossypinus, no. pos/no. tested (%) | S. hispidus, no. pos/no. tested (%) | N. floridana, no. pos/no. tested (%) |
|---|---|---|---|
| Georgia | |||
| Sapelo Island | 2/9 (22) | 1/9 (11) | — |
| St. Catherines Island | 5/113 (4) | — | — |
| Bulloch County | 12/50 (24) | 2/20 (10) | 5/14 (35) |
| St. Marys | 1/17 (6) | — | — |
| Mistletoe State Park | 2/10 (20) | 0/17 (0) | 1/8 (13) |
| Screven County | — | 2/7 (28) | — |
| Florida | |||
| Merritt Island | 3/63 (5) | 6/43 (13) | — |
| Faver-Dykes State Park | 0/12 (0) | 1/1 (100) | — |
| Amelia Island | 0/7 (0) | 1/11 (9) | — |
| Tall Timbers Res. Station | 2/38 (5) | 0/20 (0) | 0/5 (0) |
| South Carolina | |||
| Wedge Plantation | 17/26 (65) | 2/17 (12) | 8/14 (57) |
| Mt. Pleasant | 11/16 (69) | 8/10 (80) | 7/9 (78) |
| Hobcaw Barony | 10/15 (67) | — | 8/13 (62) |
| Sumter County | 2/25 (8) | 0/1 (0) | — |
| Jasper County | 2/6 (33) | — | 4/22 (18) |
| Chester County | 3/22 (14) | — | — |
| Total | 69/407 (17) | 26/178 (15) | 33/85 (39) |
A total of 128 spirochetal strains were isolated from P. gossypinus (69 isolates), N. floridana (33 isolates), and S. hispidus (26 isolates) (Table 1). All were identified as B. burgdorferi s.l. There is considerable genetic diversity among these isolates, which includes B. burgdorferi s.s., B. bissettii, B. andersonii, and possibly an undescribed genospecies. The genetic relationships of these genospecies to others found outside North America are shown in Fig. 1. Twenty-three strains isolated from P. gossypinus, S. hispidus, and N. floridana in a suburb of Charleston, SC, were all B. burgdorferi s.s. Strains MI-2, SI-1, SM-1, and SCI-2, isolated from P. gossypinus captured on Merrit Island, FL; and Sapelo Island, St. Marys, and St. Catherines Island, GA, were also B. burgdorferi s.s., as were several others (8, 9, 17). However, several different strains, some of which were isolated from P. gossypinus, S. hispidus, and N. floridana from the same geographic sites as those yielding B. burgdorferi s.s. were identified as B. bissettii. Clearly, these three rodent species harbor both B. burgdorferi s.s. and B. bissettii, but we have not yet isolated both of these genospecies from a single individual rodent. Infectivity of some genospecies to several host species is common. For example, the three main spirochete genospecies in Europe (B. burgdorferi s.s., B. afzelii, B. garinii) that cause human LD may share common rodent hosts. The genospecies B. andersonii, which is transmitted by Ixodes dentatus, appears to be an exception and is primarily restricted to the cottontail rabbit in eastern North America.
Fig. 1.
Phylogenetic tree based on DNA sequences of rrf-rrl intergenic spacer amplicons of southern and reference strains of B. burgdorferi s.l.
Infection of Reservoirs and Infectivity of Borrelia. Records of the duration of infection of B. burgdorferi in several P. gossypinus, S. hispidus, and N. floridana individuals indicate that most remain infected for life. Moreover, infection with B. burgdorferi s.s. or B. bissettii does not appear to decrease longevity of these rodents in the laboratory. Indeed, some laboratory-maintained infected rodents lived a remarkably long time and remained infected until death. Infection was confirmed periodically and at death by reisolation of spirochetes in Barbour–Stoenner–Kelly H culture medium (Table 2). All of the individuals listed in Table 2 were naturally infected when captured, and thus the ages listed do not include the time they were infected before capture. The five longest infections in P. gossypinus were >47, 48, 58, 59, and 62 months, and all remained infected with B. burgdorferi s.l. at death (Table 2). Two other infected mice lived 38 and 47 months in captivity, remained infected for at least 30 and 35 months, respectively, and probably were infected at death based on comparison with the other five mice. However, tissues from one of the two mice (CWB-59) were not cultured at death, and although tissues from the other (CWB-44) were cultured, they were beginning decomposition when the mouse was found, and no spirochetes were isolated.
Table 2. Length of B. burgdorferi s.l. infection in three rodent species based on biopsy cultures in Barbour–Stoenner–Kelly H medium.
| Host species and number | Isolate | Genospecies* | First isolated | Reisolated | Died |
|---|---|---|---|---|---|
| P. gossypinus | |||||
| SC-128A | SCSC-2 | B. b. s.s. | 4/17/95 | 09/27/97; 12/98; 03/06/99 | 03/06/99; infected |
| CM-1 | SCW-37 | B. b. s.s. | 4/21/95 | 09/27/97; 12/98; 06/06/00 | 06/06/00; infected |
| SC-168A | SCGT-4 | B. b. s.s. | 5/6/95 | 09/27/97; 12/98; 02/22/00 | 02/22/00; infected |
| SC-165A | SCGT-3 | B. b. s.s. | 5/6/95 | 09/27/97; 12/98; 04/20/99 | 04/20/99; infected |
| SC-172A | SCGT-5 | B. bis. | 5/6/95 | 09/27/97; 12/98; 03/26/00 | 03/26/00; infected |
| CWB-44 | BUL-10 | B. b. s.s. | 6/20/97 | 09/27/97; 12/98; 04/00 | 05/04/01; ear, heart kidney, spleen negative cultures |
| CWB-59 | BUL-13 | N. I. | 11/1/97 | 09/27/97; 12/98; 04/00 | 12/27/00; not cultured |
| S. hispidus | |||||
| Cotton rat3 | MI-7 | B. b. s.s. | 6/17/92 | 12/16/93 | 12/16/93; infected |
| Cotton rat11 | MI-8 | B. bis. | 6/17/92 | 02/07/94; 11/19/94 | 11/19/94; infected |
| N. floridana | |||||
| WR-3 | SCW-18 | B. bis. | 4/17/95 | 09/27/97; 12/98; 04/00; 03/20/01; 02/12/02; 11/01/02 | 11/21/02; not cultured |
| SC Wedge | SCW-63 | B. bis. | 6/1/95 | 09/27/97; 12/98; 04/00; 03/20/01; 02/12/02; 11/01/02 | Still alive 6/1/03 |
| SC-227 | SCGT-20 | B. b. s.s. | 1/1/96 | 09/27/97; 12/98; 04/00; 03/20/01; 02/12/02; 11/01/02 | 03/17/03; not cultured |
| CWB-49 | SCJ-4 | B. bis. | 7/1/97 | 09/27/97; 12/98; 04/00; 03/20/01; 02/12/02; 11/01/02 | 11/22/02; not cultured |
Identity-based restriction fragment length polymorphism B. b. s.s., B. burgdorferi s.s.; B. bis., B. bissettii; N. I., not identified.
Two S. hispidus remained infected for life and died after 19 and 30 months (Table 2). Four N. floridana woodrats remained alive and infected as of November 2002 (Table 2). One had been infected for >92 months, one >90 months, one >83 months, and one >65 months when spirochetes were reisolated. Two died shortly after reisolation of spirochetes. A third died 4 months later, and the fourth rat remained alive as of June 2003. The third rat lived for 7 years 3 months and was infected with B. burgdorferi s.s. The rat was still alive as of June 2003, is >8 years old, and is infected with B. bissettii (Table 2). These are remarkable ages for rats, suggesting that B. burgdorferi s.s. and B. bissettii are not detrimental to woodrat longevity. Moreover, most of these rodents remained infective to ticks. For example, I. scapularis larvae were allowed to feed on the two hispid cotton rats (one infected with MI-7, one with MI-8 B. burgdorferi s.l. strains), and although one rat died during tick feeding, the ticks had already become infected, as had the ticks that fed on the other rat. Thus, the rats remained infective to ticks for at least 18 and 19 months, respectively, since their captures. The fed larvae produced 17 and 9 unfed nymphs, respectively, from each cotton rat host. The 17 nymphs were placed on one white laboratory mouse and the 9 nymphs exposed to another one. Fifteen of the 17 and 6 of the 9 nymphs fed successfully on the two mice. One month later, ear biopsies from the two mice were cultured, and both mice were infected.
Another xenodiagnostic tick feeding was conducted in March 2001 to assess infectivity of infected woodrats. I. scapularis larvae were exposed to four infected woodrats. The resulting nymphs were fed on 12 naive laboratory mice, and 11 of the 12 mice became infected. A cohort of those unfed nymphs were also cultured in Barbour–Stoenner–Kelly H medium. Nymphs derived from larvae fed on woodrat WR-3 infected with B. bissettii (SCW-18) yielded 6 of 10 positive cultures; nymphs from woodrat SC Wedge infected with B. bissettii (SCW-63) produced 11 of 11 positive cultures; 9 of 11 nymphal cultures of ticks from woodrat SC-227 were infected with B. burgdorferi s.s. (SCGT-20), and nine of nine nymphal cultures from woodrat CWB-49 infected with B. bissettii (SCJ-4) were positive. Clearly, the woodrats remained not only infected with B. bissettii or B. burgdorferi s.s., but also infective to ticks for several years, probably for life.
Vector Competence. Transmission experiments involving I. scapularis and B. burgdorferi s.s. strains from the southeastern U.S. demonstrate excellent vector competency (12). Additionally, experiments indicate that this tick can also transmit B. bissettii. The closely related but usually non-human biting I. affinis also experimentally transmitted the B. burgdorferi s.s. isolate SI-1 (J.H.O., A.M.J., and C.W.B., unpublished data). Although I. minor is not currently considered a member of the Ixodes ricinus species complex, as are I. scapularis and I. affinis, it is an efficient vector of B. bissettii and B. burgdorferi s.s. (J.H.O., J. B. Phillips, C.W.B., L.G., T.L., and A.M.J., unpublished data). The prevalence of B. burgdorferi s.l. in natural populations of I. affinis and I. minor is much greater than in I. scapularis. For example, in South Carolina, 26% (19/74) of I. affinis,9%(2/23) I. minor, and 1.3% (12/864) of I. scapularis were naturally infected (19). Although I. affinis and I. minor rarely bite humans, it appears they are more important than I. scapularis as enzootic vectors of B. burgdorferi s.l. Presumably they play a role similar to that of I. spinipalpis in the western U.S. (11).
Discussion
Efficient reservoir hosts of B. burgdorferi s.l. share several characteristics. They are abundant, and a large number of them are naturally infected and serve as hosts to numerous juvenile vector competent ticks. They do not usually become resistant to repeated tick feeding. They are readily infected and remain infected and infective to competent tick vectors for long periods of time, often for life. Also, the hosts usually have limited home ranges, resulting in their remaining in tick-infected areas. P. gossypinus (cotton mouse), S. hispidus (hispid cotton rat), and N. floridana (eastern woodrat) share these characteristics.
Our data suggest that woodrats do not become resistant to repeated tick feeding. Moreover, the white-footed mouse, Peromyscus leucopus, does not become resistant nor does M. musculus (20). Additionally, we routinely use individual woodrats repeatedly to feed I. minor and I. scapularis for tick colony maintenance and have noticed no resistance to tick feeding as is commonly seen in rabbits and guinea pigs. Finally, P. gossypinus, S. hispidus, and N. floridana have limited home ranges (21–23) and, if infected, would tend to maintain spirochete foci. Data presented above clearly indicate that P. gossypinus, S. hispidus, and N. floridana are natural reservoir hosts to B. burgdorferi s.s. and B. bissettii in the southeastern U.S., and that they are attractive hosts to the vector-competent I. scapularis, I. affinis, and I. minor.
One of the remarkable discoveries of this research was the longevity of these three reservoirs. The long lives and infectivities mean that these three rodents may serve as a source of spirochetes for several cohorts of tick vectors. There are reports that spirochetemic dusky-footed woodrats (Neotoma fuscipes) remain infectious for ticks for at least 13–15 months in California (11), whereas the eastern woodrats (N. floridana) in our study lived for 65–92 months in the laboratory and were infected on capture. Two cotton rats (S. hispidus) remained infected for >19–30 months. There are reports (24) that in Europe the small wood mice Apodemus flavicollis and Apodemus sylvaticus remain infected with spirochetes their entire lives, which may be up to 40 months. This can be contrasted with the longevity of five cotton mice (P. gossypinus) in our study that lived for 47–62 months (Table 2).
The established role of I. scapularis as a vector of B. burgdorferi s.s. and the demonstrated presence of this spirochete in the region suggest that coastal sites in the southeast represent a risk for contracting LD (8, 9, 12, 17). Similarly, we found I. minor to be a relatively common tick on rodents, especially woodrats, in coastal and some adjacent regions in all three states. We made B. burgdorferi s.l. isolates from >20 I. minor larvae, nymphs, and adults. This may have epidemiological significance in light of B. burgdorferi s.s. and B. bissettii spirochete isolations also made from this tick species (8, 9). A similar situation exists regarding I. affinis, which, like I. scapularis, belongs to the “I. ricinus species complex,” several members of which are vectors of pathogenic B. burgdorferi circumglobally. Immature stages of I. affinis are relatively common on rodents in coastal regions of South Carolina and Georgia, and we have made >27 isolates of B. burgdorferi s.s. and B. bissettii from I. affinis. Of the six tick species collected from rodents captured in this study, only I. scapularis, I. minor, and I. affinis were infected with B. burgdorferi s.l. or B. bissettii.
Supplementary Material
Acknowledgments
This research was supported by grants from the National Institutes of Health and the Centers for Disease Control and Prevention to J.H.O.
Abbreviations: LD, Lyme disease; s.s, sensu stricto; s.l., sensu lato.
References
- 1.Centers for Disease Control and Prevention (2002) Morbid. Mortal. Wkly. Rep. 51, 29–31. [Google Scholar]
- 2.O'Connell, S., Granstrom, M., Gray, J. S. & Stanek, G. (1998) Zentbl. Bakteriol. 287, 229–240. [DOI] [PubMed] [Google Scholar]
- 3.Lane, R. S., Piesman, J. & Burgdorfer, W. (1991) Annu. Rev. Entomol. 36, 587–609. [DOI] [PubMed] [Google Scholar]
- 4.Barbour, A. G. & Fish, D. (1993) Science 260, 1610–1616. [DOI] [PubMed] [Google Scholar]
- 5.Donahue, J. G., Piesman, J. & Spielman, A. (1987) Am. J. Trop. Med. Hyg. 36, 92–96. [DOI] [PubMed] [Google Scholar]
- 6.Piesman, J. (2002) in Lyme Borreliosis: Biology, Epidemiology and Control, eds. Gray, J. S., Kahl, O., Lane, R. S. & Stanek, G. (CABI, Oxon, U.K.), pp. 223–249.
- 7.Jaenson, T. G. T. & Tälleklint, L. (1999) in Acarology IX Symposia, eds. Needham, G. R., Mitchell, R., Horn, D. J. & Welbourn, W. C. (Ohio Biological Survey, Columbus), Vol. 2, pp. 409–414. [Google Scholar]
- 8.Lin, T., Oliver, J. H., Jr., Gao, L., Kollars, T. M., Jr., & Clark, K. L. (2001) J. Clin. Microbiol. 39, 2500–2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin, T., Oliver, J. H., Jr., & Gao, L. (2002) J. Clin. Microbiol. 40, 2572–2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Picken, R. N., Cheng, Y., Strle, F. & Picken, M. M. (1996) J. Infect. Dis. 174, 1112–1115. [DOI] [PubMed] [Google Scholar]
- 11.Brown, R. N. & Lane, R. S. (1992) Science 256, 1439–1442. [DOI] [PubMed] [Google Scholar]
- 12.Oliver, J. H., Jr., Chandler, F. W., Jr., Luttrell, M. P., James, A. M., Stallknecht, D. E., McGuire, B. S., Hutcheson, H. J., Cummins, G. A. & Lane, R. S. (1993) Proc. Natl. Acad. Sci. USA 90, 7371–7375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clark, K. L., Oliver, J. H., Jr., Mckechnie, D. B. & Williams, D. C. (1998) J. Vect. Ecol. 23, 89–105. [PubMed] [Google Scholar]
- 14.Durden, L. A., Banks, C. W., Clark, K. L., Belbey, B. V. & Oliver, J. H., Jr. (1997) J. Parasitol. 83, 374–381. [PubMed] [Google Scholar]
- 15.Durden, L. A., Hu, R., Oliver, J. H., Jr., & Cilek, J. E. (2000) J. Vect. Ecol. 25, 222–228. [PubMed] [Google Scholar]
- 16.Durden, L. A., Klompen, J. S. H. & Keirans, J. E. (1993) J. Parasitol. 79, 283–286. [PubMed] [Google Scholar]
- 17.Oliver, J. H., Jr., Clark, K. L., Chandler, F. W., Jr., Lin, T., James, A. M., Banks, C. W., Huey, L. O., Banks, A. R., Williams, D. C. & Durden, L. A. (2000) J. Clin. Microbiol. 38, 120–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Swofford, D. L. (2002) paup*: Phylogenetic Analysis Using Parsimony (*and other methods) (Sinauer, Sunderland, MA), Ver. 4.
- 19.Clark, K. L., Oliver, J. H., Jr., James, A. M., Durden, L. A. & Banks, C. W. (2002) J. Med. Entomol. 39, 198–206. [DOI] [PubMed] [Google Scholar]
- 20.Galbe, J. & Oliver, J. H., Jr. (1992) J. Med. Entomol. 29, 774–783. [DOI] [PubMed] [Google Scholar]
- 21.Cameron, G. N. & Spencer, S. R. (1981) Mamm. Species 158, 1–9. [Google Scholar]
- 22.Wiley, R. W. (1980) Mamm. Species 139, 1–7. [Google Scholar]
- 23.Wolfe, J. L. & Linzey, A. V. (1977) Mamm. Species 70, 1–5. [Google Scholar]
- 24.Gern, L., Siegenthaler, M., Hu, C. M., Leuba-Garcia, S., Humair, P. F. & Moret, J. (1994) Eur. J. Epidemiol. 10, 75–80. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.