Abstract
Does the neonate's brain have left hemisphere (LH) dominance for speech? Twelve full-term neonates participated in an optical topography study designed to assess whether the neonate brain responds specifically to linguistic stimuli. Participants were tested with normal infant-directed speech, with the same utterances played in reverse and without auditory stimulation. We used a 24-channel optical topography device to assess changes in the concentration of total hemoglobin in response to auditory stimulation in 12 areas of the right hemisphere and 12 areas of the LH. We found that LH temporal areas showed significantly more activation when infants were exposed to normal speech than to backward speech or silence. We conclude that neonates are born with an LH superiority to process specific properties of speech.
Two models attempt to account for the origin of the left-hemisphere (LH) dominance for speech. The first model assumes that, at birth, the LH displays superiority in processing all acoustic signals (1). The second postulates that neonates are endowed with specific structures to processes speech signals in the LH (2). Both models assume an LH superiority at birth. However, only the second model postulates that the LH superiority is specific for speech and that it may be intrinsically related to the emergence of the language faculty. During development, the infant's brain grows and matures, and its functional organization changes, even if its gross anatomy displays striking similarities to that of the adult (3) from the start. The association of language with the LH may arise as a consequence of language acquisition, or, alternatively, this association may reflect an innate disposition of certain areas of the brain for language. Several behavioral studies have focused on this issue. One study (4) measured foot-kicking responses and observed behaviors that suggested an LH superiority for speech stimuli as compared with other auditory stimuli only hours after birth. Another study reported a right ear advantage for speech stimuli with 3-month-old infants by using the orienting response (5). An additional study used the nonnutritive sucking response to test 2-week-old infants and found a right ear advantage for speech but not for other auditory stimuli (6). A recent study with older infants reports that, as soon as babbling sets in, the mouth tends to rise toward the right side of the face, suggesting an underlying LH superiority. This asymmetry is absent during nonlinguistic vocal gestures (7). These behavioral studies suggest that speech stimuli presented to prelinguistic infants result in greater LH involvement and that an LH superiority is apparent as soon as the first language-like productions begin. Nonetheless, behavioral methods have limitations: neonates often fail to complete the tests because of fussing or crying. The advent of brain-imaging techniques has made it possible to test young infants even when they fail to make overt responses. Furthermore, imaging methods link behavioral observations to their underlying brain mechanisms.
Event-related potentials have been used to advance our understanding of speech processing in very young infants (8, 9). Event-related potentials have an excellent time resolution but a weak spatial resolution. Nonetheless, event-related potentials have provided evidence that sleeping neonates can discriminate a phonetic change despite a change of speaker (10). A recent functional MRI (fMRI) study with 3-month-old infants (11) found that auditory stimuli give rise to greater activity in the LH superior temporal lobe as compared with silence. However, the authors failed to find different activation for normal forward speech (FW) as compared with reversed backward speech (BW) in the temporal lobe. Rather, they report a larger activation for FW as compared with BW in posterior areas of the LH. This study has contributed to our understanding of the development of the LH dominance for speech. Yet, we still have to assess whether the neonate brain reacts to speech like that of 3-month-old infants. A number of behavioral studies have documented that neonates process FW and BW differently (12, 13). Infants acquire several properties of the surrounding language during the first 3 months of life (14), which could account for the findings reported in the fMRI study quoted above. Thus, we still have to elucidate whether there is an LH dominance for speech in neonates.
Our study uses optical topography (OT) to test whether neonates display a LH superiority for FW and, in addition, whether an activation difference exists for FW vs. BW. OT is a silent noninvasive imaging technique, ideally suited to test precocious auditory competence. A precocious processing asymmetry between normal and backward speech could document that humans are born with brain areas dedicated, in part, to speech processing, thus clarifying the status of speech perception at birth, which previous studies have left unresolved.
Methods
Participants. Fourteen full-term healthy Italian neonates ranging in age from 2 to 5 days participated in this study (mean age 2.7 days). Two neonates were excluded because they cried and/or failed to complete the experimental protocol. All infants had an Apgar score ≥9 at 1 and 3 minutes after birth. All neonates were tested while sleeping. The ethics committee of the Burlo Garofolo Hospital in Trieste, where all of the testing was conducted, granted permission. When the parents had received all of the relevant information, we asked them to sign a consent form.
Stimulus Materials. We recorded speech samples from two Italian mothers (whose infants did not participate in this experiment) while they recited children's stories using infant-directed speech, a speech style that adults use when speaking to infants. Normally, adults using infant-directed speech speak at a slower rate and increase the average pitch. In some cases, they also tend to enhance their prosodic excursions. Stories were edited into sequences of 15 s (±1 s) each. A phonologist verified that all sequences were well formed prosodic units. The mean intensity of the sentences was equalized. A speech editor that time-reversed the FW utterances generated the stimuli for the BW condition. Each block used sequences from only one speaker.
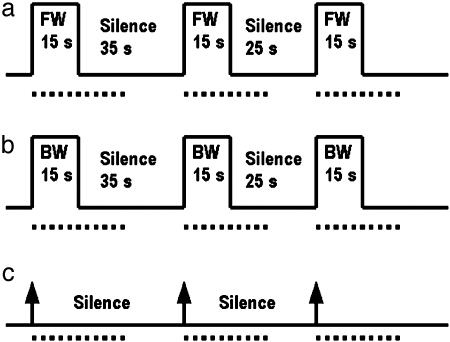
Testing Protocol. Neonates were tested while they slept in a silent room. Tests consisted of 10 blocks per condition, presented in random order: FW, BW, and silent control condition (SIL) (see Fig. 1). Each block contained 15 s of FW followed by 25–35 s of silence, 15 s of BW followed by 25–35 s of silence, or 15 s of silence followed by 25–35 s of silence. The varying durations of the silent periods were introduced to avoid synchronization between stimuli occurrences and spontaneous oscillations. Spontaneous oscillations are periodic variations in the OT signal due to the autonomic regulation of cerebral blood flow (15). BW is an optimal control for FW because it matches the latter in duration, pitch, and intensity. Many segments sound alike in both FW and BW. However, the human vocal tract cannot produce some backward sequences, in particular, the ones corresponding to backward aspirated stops. Behavioral studies have demonstrated that neonates cannot discriminate a pair of languages when the utterances are reversed, whereas they can when they hear normal utterances (13). In the SIL, the infant received no auditory stimuli, but marks were sent to the OT device corresponding to the labels that would have signaled auditory stimuli in the two other conditions.
Fig. 1.
Testing protocol. Neonates were tested by using 10 blocks per experimental condition: FW (a), BW (b), and SIL (c). Only three of 10 blocks are illustrated for each condition. A block begins with the presentation of 15 s of the stimulus. A period of silence of varying duration (from 25 to 35 s) follows the stimulation period. In c, the epochs that correspond to stimulation epochs in conditions FW and BW are silent. The arrows indicate the onset of the periods of 15 s used to align SIL with FW and with BW during the analyses. The periods used for statistical analyses start with the onset of the block and finish 30 s later (indicated by the dotted line).
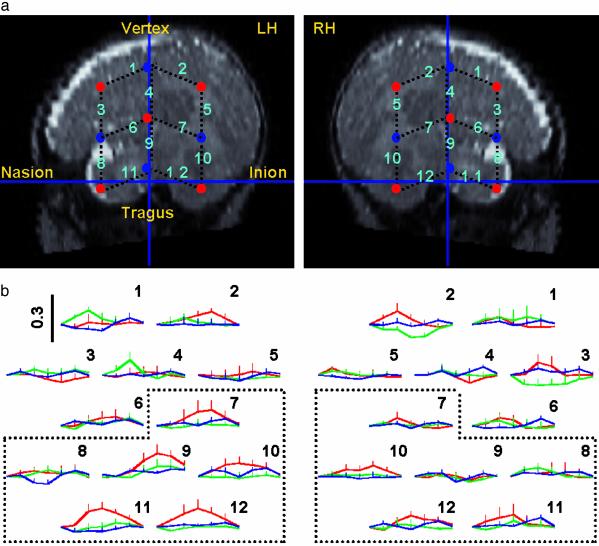
Experimental Procedure. Neonates were tested in their cribs. A silicon holder (hereafter called a probe) kept the optical fibers in place (see Fig. 2a). We used two probes, each containing nine optical fibers of 1 mm in diameter. One probe was placed over the right hemisphere (RH) and the other over the LH. Probes were positioned to maximize the likelihood of monitoring perisylvian areas. Of the nine fibers per probe, five were emitters and four were detectors. Fibers were placed 3 cm apart, providing 12 recording sites per hemisphere (hereafter called channels). Each channel corresponds to the central zone of the light path between each adjacent emitter–detector fiber pair. The probes were placed by using skull landmarks. First, the vertex was determined as the site where the midpoint of a line going from the nasion to the inion intersects the midpoint of another line going from the left to the right preauricular lobule. When the vertex had been determined, the two probes were positioned to ensure that channels 4 and 9 coincided with the imaginary line going from the vertex to the preauricular lobule and that channels 11 and 12 coincided with the imaginary line going from the inion to the nasion. When the probes were positioned, the experimenter made sure that the fibers touched the neonate's scalp. The OT device detects automatically whether the contact is adequate to measure emerging photons.
Fig. 2.
Positioning of the OT probes and observed results. (a) OT channels projected on an MR image of a 2-month-old infant. Red dots correspond to emitter and blue dots to detector optical fibers. The numbers on the black dotted lines, between adjacent emitter–detector pairs of fibers, correspond to the channels from which changes in Hb concentration were estimated. Indicated skull landmarks (inion, nasion, tragus, and vertex) were used to place the probes. (b) The numbers above the plots correspond to channel numbers in a. The plots show the grand average of the mean of total Hb (mmol·mm) for successive 5-s windows. The first window begins 5 s before the onset of a block. The vertical black line in channel 1 of the LH indicates the range of total Hb concentration in mmol·mm valid for all of the channels. Total Hb is plotted in red for FW, in green for BW, and in blue for SIL. Ascending bars indicate SDs. The six channels enclosed within dotted lines (7–12) cover the temporal regions below the Sylvian fissure (lower channels). Channels 1–6 were placed over the frontoparietal regions above the Sylvian fissure (upper channels).
OT Recording. OT estimates change in cerebral blood volume and oxygen saturation. A number of papers provide a detailed description of the principles underlying OT (16–18). Previous studies suggest that the properties of the vascular response measured using OT are comparable to those described for the BOLD (blood oxygen level dependence) effect in fMRI (19). The Hitachi ETG-100 OT device records simultaneously from 24 channels on the cortex. Channels mostly measure vascular changes from the surface of the cortex, that is, 2–3 cm below the scalp. The ETG-100 emits infrared light at two wavelengths, 780 and 830 nm, through the fibers. The intensity of each wavelength is modulated at different frequencies ranging from 1 to 6.5 kHz, and the total power output per fiber is 0.7 mW. The reflected light is sampled once every 100 ms and is separated into two modulated signals, one for each wavelength, by a number of corresponding lock-in amplifiers. After analog-to-digital conversion, the signals are transferred to a computer. These measures make it possible to estimate the changes in the concentration of oxy-Hb, deoxy-Hb, and total Hb in response to stimulation. Given the wavelengths used by the ETG-100 model, oxy-Hb and total-Hb estimations are more precise than the estimation of deoxy-Hb.
Data Analysis. Our analysis focused on the variation of total Hb, which estimates the changes in the cerebral blood volume. Total Hb was calculated from the absorption of the two wavelengths mentioned above. To remove components originating from slow fluctuations of cerebral blood flow and heartbeat noise, the total Hb signal was bandpass filtered between 0.02 and 1 Hz. Moreover, all blocks that had been affected by movement artifacts were removed after detecting rapid changes in total Hb concentration (signal variations >0.1 mmol·mm over two consecutive samples). Thereafter, a first-degree baseline fit was estimated for each channel in nonrejected blocks. The fit was computed between the mean of the 5 s before the onset of the block and the mean of the 5 s between the 25th and 30th s after the onset of the block. For each neonate, the mean change in concentration of total Hb over the 30 s after the onset was calculated for each condition and for each channel. These measures were used to carry out the statistical analyses. Additional analyses of shorter periods of the response were conducted for channels over the temporal regions. Statistical analyses were carried out by using three-way repeated measures ANOVA: condition (FW, BW, and SIL) × hemisphere (LH and RH) × group of channels (upper channels and lower channels). The two groups of channels (upper and lower) were used to identify relatively extensive areas of brain activation. Upper channels, 1–6, covered the superior half of the frontoparietal cortex above the Sylvian fissure, whereas lower channels, 7–12, covered most of the temporal cortex under the Sylvian fissure (Fig. 2a). Additional ANOVAs were conducted to study the activation on each independent channel (12 channels in the LH and 12 channels in the RH).
Results
Our results focus on the variations in the concentration of total Hb as illustrated in Fig. 2b. We observed a main effect of hemisphere (F1,11 = 9.17, P < 0.012) due to a significantly greater activity in the LH than in the RH. A post hoc analysis showed that this effect was due to a greater activation of the LH only during the FW condition.
In fact, during FW, the concentration of total Hb was significantly greater in the LH than in the RH (F1,11 = 11.03, P < 0.007); no significant differences by hemisphere were observed during the BW or the SIL conditions. The response to FW was mostly localized in the lower channels overlying the temporal regions (main effect of the group of channels: F1,11 = 12.68, P < 0.004; condition x group of channels interaction: F1.41,22 = 5.06, P < 0.029). In the lower channels of the LH, the concentration of total Hb was significantly higher for FW than for either BW or SIL (F1,11 = 8.73, P < 0.013 and F1,11 = 8.56, P < 0.014, respectively). No significant differences by condition or hemisphere were found in the upper channels or in the lower channels of the RH. The analysis of independent channels showed that in channel 9 of the LH, the concentration of total Hb was significantly greater than in channel 9 of the RH only during FW (F1,11 = 7.59, P < 0.019). Moreover, in channel 11 of the LH, the concentration of total Hb was significantly higher during FW than during BW or SIL (F1,11 = 12.88, P < 0.004), whereas there were no significant differences by condition in channel 11 of the RH. Finally, if we restrict the comparisons to the period from 10 to 20 s after the onset of the block (which coincides with the plateau of the vascular response), in channel 9 the response was significantly higher in the LH compared with the RH (F1,11 = 9.70, P < 0.01), and it was significantly higher during FW compared with SIL (F1,11 = 5.69, P < 0.036), suggesting that left temporal areas were particularly responsive to FW speech.
Discussion
Many studies have established a connection between language and the LH. Since Broca in 1861 (20), neurolinguists have acknowledged this connection. Most of the significant data until the advent of brain imaging, however, came from the observation of patients. One of the first imaging studies of speech comprehension in normal adults showed that the perisylvian LH is more activated than the homologous RH areas in volunteers who listen passively to simple stories in their native language (21). A similar fMRI study (22) found greater LH activation in adult Italian volunteers while they listened to stories in an unknown language (Japanese) as compared to the same stories played backwards. Another study (23) used fMRI to explore how first and second languages are cortically represented in the brains of bilinguals. In most participants, the LH was dominant for the first and second languages.
LH superiority is not only restricted to perception of speech but is also observed during speech production. Indeed, it has been reported that a small part of the LH insula is responsible for the smooth production of speech (24). A large study of patients with and without apraxic symptoms showed that only lesions in a particular region of the LH insula invariably result in speech apraxia.
It is not clear whether language learning strengthens the LH dominance for speech or whether the LH is endowed with specific abilities to process speech that render it ideal for learning language. A case study (25) showed that a child who had previously been unable to acquire language had become able to make exceptional progress at the age of 9 when he began recovering from the surgical resection of his LH, which had been damaged since birth. This observation supports the notion that, under some conditions, speech processing can migrate from the dominant to the contralateral hemisphere. Nonetheless, this does not contradict the notion of an initial asymmetry.
Our OT study provides clear evidence that, at birth, the human brain is functionally organized to process speech in the LH but not matched reversed utterances. Indeed, adults' LH is more responsive to FW than to BW when tested with an unknown language (22). This result is similar to our own observation with neonates tested with OT that the LH of the neonates responds more to FW than to BW and to SIL. These observations suggest that some properties of FW are essential to activate LH areas that could be involved in adult speech processing. The aforementioned clear asymmetry reported in our paper implies that humans are born with a brain organization geared to detect speech signals and pay attention to utterances produced in their surroundings.
Our observations also confirm that OT is a useful technique for exploring the organization of the neonate brain. This is an important result because OT is a noninvasive and silent procedure that is well suited to study auditory competences in neonates and in young infants. Moreover, our results are quite similar to the fMRI results obtained with older infants (11). Both OT and fMRI studies have found an LH superiority in the processing of sound stimuli. The studies, however, failed to find similar areas responding to FW as compared with BW. In the fMRI study with 3-month-old infants, greater activations for FW as compared with BW were observed in the left angular gyrus and the left mesial parietal cortex. In the OT study with neonates, activations were observed in temporal areas of the LH like one usually observes with adults. The absence of a left mesial parietal response in the neonates could be due to limitations of the OT system to measure changes in Hb concentration in depths greater than 3 cm from the scalp. However, the contrasts between the OT and the fMRI studies may also reflect postnatal developments that distinguish neonates from 3-month-old infants. Last but not least, the fact that fMRI operates under noise while OT operates silently may also explain some of the differences between the fMRI and OT studies. The single most important finding of this study is that it provides the demonstration that the neonate brain responds specifically to normal speech only after a few hours of experience with speech signals outside the womb.
Acknowledgments
We thank all of the personnel of Istituto di Ricovero e Cura a Carattere Scientifico Burlo Garofolo, Trieste, Italy. Drs. U. de Vonderweid, E. Arbustini, and R. Forleo were very helpful at various stages of this research. We particularly thank Nurse Carla Pavan for her valuable assistance in the recruitment of neonates. Our gratitude also goes to the parents and the infants for their interested participation. We thank Richard Aslin, Luca Bonatti, Franck Ramus, Doug Saddy, Tim Shallice, and Mohinish Shukla for helpful comments, and Susana Franck, Valerie Lesk, Judit Gervain, and Mohinish Shukla for assistance in the preparation of this manuscript. We thank Mr. Noriyoshi Ichikawa and Fumio Kawaguchi of Hitachi Medical Corporation for technical assistance. This work was supported by a grant from Ministero dell'Universitá e della Ricerca Scientifica e Tecnologica to the Istituto di Ricovero e Cura a Carattere Scientifico Burlo Garofolo, a grant from the Regione Friuli Venezia Giulia, a grant from Ministerio de Planificación y Cooperación de Chile to Marcela Peña, and by Human Frontiers Science Program Grant RGP 0068/2002-C.
Abbreviations: LH, left hemisphere; OT, optical topography; RH, right hemisphere; fMRI, functional MRI; FW, forward speech; BW, backward speech; SIL, silent control condition.
References
- 1.Bradshaw, J. L. & Nettleton, N. (1983) Human Cerebral Asymmetry (Prentice–Hall, Scarborough, ON, Canada).
- 2.Mehler, J. & Dupoux, E. (1994) What Infants Know (Basil Blackwell, Cambridge, MA).
- 3.Neville, H. J. & Bavelier, D. (2000) in The New Cognitive Neurosciences, ed. Gazzaniga, M. S. (MIT Press, Cambridge, MA), pp. 83–89.
- 4.Segalowitz, S. J. & Chapman, J. S. (1980) Brain Lang. 9, 281–288. [DOI] [PubMed] [Google Scholar]
- 5.Best, C. T. (1988) in Brain Lateralization in Children, eds. Molfese, D. L. & Segalowitz, S. J. (Guilford, New York), pp. 3–5.
- 6.Bertoncini, J., Morais, J., Bijeljac-Babic, R., McAdams, S., Peretz, I. & Mehler, J. (1989) Brain Lang. 37, 591–605. [DOI] [PubMed] [Google Scholar]
- 7.Holowka, S. & Petitto, L. A. (2002) Science 297, 1515. [DOI] [PubMed] [Google Scholar]
- 8.Cheour, M., Ceponiene, R., Lehtokovski, A., Luuk, A., Allik, J., Alho, K. & Näätänen, R. (1998) Nat. Neurosci. 1, 351–353. [DOI] [PubMed] [Google Scholar]
- 9.Dehaene-Lambertz, G. & Dehaene, S. (1994) Nature 370, 292–295. [DOI] [PubMed] [Google Scholar]
- 10.Dehaene-Lambertz, G. & Peña, M. (2001) NeuroReport 12, 3155–3158. [DOI] [PubMed] [Google Scholar]
- 11.Dehaene-Lambertz, G., Dehaene, S. & Hertz-Pannier, L. (2002) Science 298, 2013–2015. [DOI] [PubMed] [Google Scholar]
- 12.Mehler, J., Jusczyk, P., Lambertz, G., Halsted, N., Bertoncini, J. & Amiel-Tison, C. (1988) Cognition 29, 143–178. [DOI] [PubMed] [Google Scholar]
- 13.Ramus, F., Hauser, M. D., Miller, C., Morris, D. & Mehler, J. (2000) Science 288, 349–351. [DOI] [PubMed] [Google Scholar]
- 14.Jusczyk, P. W. (1997) The Discovery of Spoken Language (MIT Press, Cambridge, MA).
- 15.Diehl, R. R., Linden, D., Lucke, D. & Berlit, P. (1998) Clin. Auton. Res. 8, 7–12. [DOI] [PubMed] [Google Scholar]
- 16.Chance, B., Zhuang, Z., Chu, U., Alter, C. & Lipton, L. (1993) Proc. Natl. Acad. Sci. USA 90, 2660–2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jobsis, F. F. (1977) Science 198, 1264–1267. [DOI] [PubMed] [Google Scholar]
- 18.Maki, A., Yamashita, Y., Ito, Y., Watanabe, E., Mayagani, Y. & Koizumi, H. (1995) Med. Phys. 22, 1997–2005. [DOI] [PubMed] [Google Scholar]
- 19.Strangman, G., Culver, J. P., Thompson, J. H. & Boas, D. A. (2002) NeuroImage 17, 719–731. [PubMed] [Google Scholar]
- 20.Broca, P. (1861) Bull. Soc. Anat. (Paris) 6, 330. [Google Scholar]
- 21.Mazoyer, B. M., Dehaene, S., Tzourio, N., Frak, V., Murayama, N., Cohen, L., Lévrier, O., Salamon, G., Syrota, A. & Mehler, J. (1993) J. Cognit. Neurosci. 5, 467–479. [DOI] [PubMed] [Google Scholar]
- 22.Perani, D., Dehaene, S., Grassi, F., Cohen, L., Cappa, S., Dupoux, E., Fazzio, F. & Mehler, J. (1996) NeuroReport 7, 2439–2444. [DOI] [PubMed] [Google Scholar]
- 23.Dehaene, S., Dupoux, E., Mehler, J., Cohen, L., Paulesu, E., Perani, D., van de Moortele, P.-F., Léhericy, S. & LeBihan, D. (1997) NeuroReport 8, 3809–3815. [DOI] [PubMed] [Google Scholar]
- 24.Dronkers, N. F. (1996) Nature 384, 159–161. [DOI] [PubMed] [Google Scholar]
- 25.Vargha-Khadem, F., Carr, L. J., Isaacs, E., Brett, E., Adams, C. & Mishkin, M. (1997) Brain 120, 159–182. [DOI] [PubMed] [Google Scholar]