Abstract
Calsequestrin is a high-capacity Ca-binding protein expressed inside the sarcoplasmic reticulum (SR), an intracellular Ca release and storage organelle in muscle. Mutations in the cardiac calsequestrin gene (CSQ2) have been linked to arrhythmias and sudden death. We have used Ca-imaging and patch-clamp methods in combination with adenoviral gene transfer strategies to explore the function of CSQ2 in adult rat heart cells. By increasing or decreasing CSQ2 levels, we showed that CSQ2 not only determines the Ca storage capacity of the SR but also positively controls the amount of Ca released from this organelle during excitation–contraction coupling. CSQ2 controls Ca release by prolonging the duration of Ca fluxes through the SR Ca-release sites. In addition, the dynamics of functional restitution of Ca-release sites after Ca discharge were prolonged when CSQ2 levels were elevated and accelerated in the presence of lowered CSQ2 protein levels. Furthermore, profound disturbances in rhythmic Ca transients in myocytes undergoing periodic electrical stimulation were observed when CSQ2 levels were reduced. We conclude that CSQ2 is a key determinant of the functional size and stability of SR Ca stores in cardiac muscle. CSQ2 appears to exert its effects by influencing the local luminal Ca concentration-dependent gating of the Ca-release channels and by acting as both a reservoir and a sink for Ca in SR. The abnormal restitution of Ca-release channels in the presence of reduced CSQ2 levels provides a plausible explanation for ventricular arrhythmia associated with mutations of CSQ2.
In cardiac muscle, contractile activation is mediated by Ca influx through plasmalemmal-voltage-dependent Ca channels activating Ca-sensitive Ca-release channels/ryanodine receptors (RyRs) in the sarcoplamic reticulum (SR). This mechanism is known as Ca-induced Ca release (CICR) (1, 2). For relaxation to occur, Ca release must stop, and the released Ca must be cleared from the cytosol. Termination of CICR appears to involve changes in the functional activity of the RyR channel, which is caused by the decline of [Ca] in the SR lumen by a mechanism termed luminal Ca-dependent deactivation (3). After termination of release, most of the Ca is resequestered to the SR (2). Because of the possibility that released Ca can activate further Ca release, the CICR process is intrinsically unstable and prone to arrhythmogenic spontaneous Ca oscillations. Spontaneous Ca waves can induce oscillations of the membrane potential, known as delayed after-depolarizations or DADs, which are underlying causes of triggered arrhythmia (4–7). Thus, tight control of CICR is essential to normal cardiac function.
It has been proposed that the SR luminal Ca-binding protein calsequestrin (CSQ) plays an important role in SR function by providing a reservoir for Ca and possibly by serving as a luminal Ca sensor for RyR (for reviews, see refs. 8 and 9). Biochemical studies showed that cardiac CSQ (CSQ2) can bind ≈40 Ca ions per molecule with a Kd ≈ 0.6 mM (10), undergoes major conformational changes on Ca binding (10, 11), and interacts with RyR by means of intermediate linker proteins, such as triadin and junctin (12, 13). However, the data presented to date regarding the physiological function of CSQ2 in the setting of intact cells are somewhat limited, and some findings are conflicting. The functional role of CSQ2 in situ has been studied in transgenic mice overexpressing CSQ2 (14, 15). These studies showed that overexpression of CSQ2 at levels up to 10- to 20-fold higher than the endogenous protein increased SR Ca storage capacity. However, the luminal Ca was not accessible for release, resulting in depressed CICR. On the other hand, we recently demonstrated that loading of the SR with low-affinity Ca buffers that possess Ca-binding properties similar to those of CSQ2 enhanced both SR Ca content and active SR Ca release (3). These results suggest that enhanced buffering by itself should enhance rather than decrease Ca flux through the SR Ca-release channels. One potential reason for these conflicting results is that prolonged, high-level overexpression of CSQ2 in transgenic mice may have negatively affected the activity of the RyRs or the activity or expression of other proteins needed for efficient calcium efflux.
Recently, it was reported that mutations of the human CSQ2 gene are associated with certain forms of hereditary ventricular arrhythmias and sudden death, evoked by β-adrenergic stimulation and exercise [catecholaminergic polymorphic ventricular tachycardia (CPVT)] (16, 17). However, our limited knowledge of CSQ function makes it difficult to understand how altered expression or deranged Ca-binding properties of this protein, caused by these mutations, can pathogenetically determine the functional abnormalities characteristic of CPVT. In the present study we have used adenovirally mediated gene transfer strategies to specifically increase or decrease the levels of CSQ2 in adult rat cardiac myocytes. The consequences of altered expression of CSQ2 on both local and global Ca signaling have been determined with the use of Ca-imaging and patch-clamp methods.
Materials and Methods
Construction of Recombinant Adenoviruses (Ad). The full-length canine CSQ2 coding sequence (GenBank accession no. J03766) was amplified by PCR and inserted into the pSHUTTLE vector (Clontech) in both the sense and antisense orientations. A CSQ2 cDNA encoding a truncated protein was also constructed in pSHUTTLE by changing the codon for amino acid 71 to a stop codon. Each CSQ2-coding region was transferred into the Adeno-X viral DNA, and recombinant adenoviruses were generated according to the instructions of the manufacturer (Clontech).
Ad Gene Transfer. Ventricular myocytes were obtained from adult male Sprague–Dawley rat hearts by enzymatic dissociation (3). After isolation, the cells were resuspended in serum-free medium 199 containing 25 mM NaHCO3, 5 mM creatine, 5 mM taurine, 10 units/ml penicillin, 10 μg/ml streptomycin, 10 μg/ml gentamicin (all Sigma), pH 7.3, and plated at a density of ≈0.5 × 104 rod-shaped cells per cm2 on laminin-coated (20 μg/ml, Sigma) glass coverslips. The cells were infected with Ad at a multiplicity of infection of 100 and maintained in a CO2 incubator for 48–56 h at 5% CO2/95% air and 37°C.
Western Blotting and Immunocytochemistry. Levels of CSQ2, cardiac SR Ca-ATPase (SERCA2a), and phospholamban proteins were determined by immunoblot analysis. Cell lysate proteins (10 μg) were subjected to SDS/8 or 12% PAGE, blotted onto poly(vinylidene difluoride) membranes (Santa Cruz Biotechnology), and probed with antibodies specific for CSQ2 (1:2,500; PA1–913, Affinity Bioreagents, Golden, CO), SERCA2 (1:1,000; sc-8094, Santa Cruz Biotechnology), or phospholamban (1:1,500; 05-205, Upstate Biotechnology, Lake Placid, NY). For immunocytochemistry, myocytes were fixed on glass coverslips with 4% paraformaldehyde and permeabilized with 0.1% Triton X-100/PBS (pH 7.2) containing 1% BSA. Specimens were incubated with anti-CSQ2 antibody (1:2,000) followed by fluorescein-conjugated, highly cross-adsorbed goat anti-rabbit IgG (1:200, Molecular Probes).
Electrophysiological Recordings. Whole-cell patch-clamp recordings of transmembrane ionic currents were performed with an Axopatch 200B amplifier (Axon Instruments, Foster City, CA). From a holding potential of –50 mV, 400-ms voltage pulses were applied at 1-min intervals to specified membrane potentials. In some experiments, the cells were stimulated periodically (2 Hz) in the current clamp configuration. The external solution contained 140 mM NaCl, 5.4 mM KCl, 1.0 mM CaCl2, 0.5 mM MgCl2, 10 mM Hepes, 5.6 mM glucose, and 0.02 mM tetrodotoxin, pH 7.3. Micropipettes made from borosilicate glass (Sutter Instruments, Novato, CA; 1- to 3-MΩ resistance) were filled with a solution that contained 90 mM cesium aspartate, 50 mM CsCl, 3 mM Na2ATP, 3.5 mM MgCl2, 10 mM Hepes, and 0.05 mM Fluo-3 potassium salt, pH 7.3.
Confocal Ca Measurements. Cells were imaged with a Bio-Rad Laser Scanning Confocal System (Bio-Rad MRC-1024ES interfaced to an Olympus IX-70 inverted microscope) equipped with an Olympus ×60 1.4 numerical aperture oil immersion objective (3). Fluo-3 was excited by light at 488 nm, and the fluorescence was acquired at wavelengths >515 nm in the line-scan mode of the confocal system at a rate of 2 or 6 ms per scan. Ca spark parameters were quantified with a detection/analysis computer algorithm (3).
Results
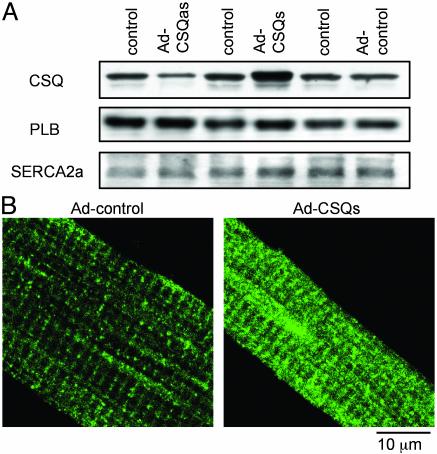
To specifically overexpress or reduce the expression of CSQ in adult rat ventricular myocytes, recombinant Ad were constructed that contained the full-length coding region of canine CSQ2 (18) in either the sense (Ad-CSQs) or antisense (Ad-CSQas) orientation. A third recombinant Ad containing the CSQ2 coding sequence with a stop codon inserted after amino acid 71 was used as a control for viral infection (Ad-control). Infection rates were assessed with an adenovirus containing the coding region for GFP and were consistently high (95 ± 4%, n = 6) at a multiplicity of infection of 100. Expression of both endogenous and recombinant proteins was examined in infected myocytes by Western blotting (Fig. 1A). CSQ2 levels were significantly elevated in cells infected with Ad-CSQs (referred to as CSQs) (3.9 ± 1.2-fold, n = 3) and significantly reduced in cells infected with Ad-CSQas (referred to as CSQas) (2.7 ± 0.2-fold, n = 3). CSQ2 levels were unchanged in cells infected with Ad-control, and both SERCA2a and phospholamban protein levels were similar in all transfections. CSQ2 targeting to SR cisternae was unchanged by overexpression of CSQ2, as judged by immunofluorescence with anti-CSQ2 antibodies (Fig. 1B). Thus, it appears that infected myocytes represent a controlled environment in which to assess the consequences of elevated or reduced CSQ2 levels on cardiac myocyte function.
Fig. 1.
Expression and localization of CSQ2 in myocytes infected with control, CSQ2 sense (CSQs), and CSQ2 antisense (CSQas) adenoviral vectors. (A) Representative immunoblots detecting CSQ, phospholamban (PLB), and SERCA2a in myocytes infected with Ad-control, Ad-CSQs, and Ad-CSQas. (B) Immunofluorescence localization of CSQ2 in myocytes infected with Adcontrol and Ad-CSQs vectors. All measurements were performed 48–56 h after infection of myocytes with the adenoviral constructs.
Applications of 10 mM caffeine were used to assess changes in the total Ca storage capacity of the SR in CSQs and CSQas myocytes. The relative amounts of Ca released from the SR by caffeine were inferred from changes in Fluo-3 fluorescence and from Na/Ca exchange current (INCX) in myocytes dialyzed with Fluo-3. The amplitude of caffeine-induced Ca transients was increased ≈2-fold in CSQs myocytes and decreased to ≈70% of control in CSQas myocytes (Fig. 2A). In addition, the integral of INCX density was 2.2-fold higher in CSQs cells, and it decreased to 52% of control in CSQas cells. These obvious changes in SR Ca content are consistent with the altered levels of CSQ2 protein expression in CSQs and CSQas myocytes, respectively.
Fig. 2.
Effects of increased and reduced CSQ2 levels on SR Ca content on caffeine- and ICa-induced Ca transients in rat ventricular myocytes. (A) Caffeine-induced intracellular Ca transients (upper traces) and INCX (lower traces) in myocytes infected with Ad-control, Ad-CSQs, and Ad-CSQas vectors. The average amplitudes (F/F0) of caffeine-induced Ca transients were 2.9 ± 0.3, 5.4 ± 0.6, 2.0 ± 0.3; integrals of INCX density were 0.54 ± 0.08, 1.22 ± 0.12, and 0.28 ± 0.05 pC/pF, for Ad-control, Ad-CSQs, and Ad-CSQas, respectively (n = 6–10). (B) Recordings of Ca transients (top traces) along with their first derivative (dF/dt, middle traces) and recordings of ICa (bottom traces) in cardiomyocytes infected with Ad-control, Ad-CSQs, and Ad-CSQas vectors. (C and D) Voltage dependence of Ca transients (C) and ICa (D) in myocytes infected with Ad-control, Ad-CSQs, and Ad-CSQas vectors (n for each point ranged from 6 to 10). The half-time decays of ICa at 0mV (t1/2) were 18 ± 4, 20 ± 6, and 17 ± 5 ms, n ≥ 6, for control, CSQs, and CSQas, respectively. All measurements were performed 48–56 h after infection of myocytes with the Ad constructs.
Simultaneous measurements of L-type Ca current (ICa) and Ca transients in response to depolarizations to various membrane potentials were performed to characterize the functional state of excitation–contraction coupling in CSQs and CSQas myocytes. The magnitude and duration of Ca transients were dramatically increased in CSQs myocytes and dramatically reduced in CSQas myocytes, as compared with control (Fig. 2 B Top and C). Likewise, the duration of the rising phase of the Ca transients was increased in CSQs myocytes (46 ± 8 ms at 0 mV, n = 8) and decreased in CSQas myocytes (16 ± 3 ms, n = 6), as compared with control (25 ± 3 ms, n = 10). The amount of Ca released from the SR in response to a given Ca stimulus is a function of both the rate and duration of Ca release. The maximum rate of release is determined primarily by the kinetics of activation, whereas the duration of release is mainly a reflection of the mechanisms responsible for inactivation of the release process. The altered rise times of Ca transients suggest that the duration of Ca-release fluxes underlying these signals were altered, i.e., prolonged or shortened, in CSQs and CSQas myocytes, respectively. The first derivative of Ca transients in Fig. 2B, middle traces, reveals that the changes in overall size of Ca transients were not accompanied by alterations in the maximal rate of release in CSQs and CSQas myocytes. Thus, changes in the duration of Ca release rather than alterations in Ca activation kinetics apparently were the main consequence of manipulating the amount of CSQ2 in myocytes. No significant change occurred in the parameters of ICa, the Ca trigger for SR Ca release, in myocytes with increased or reduced expression of CSQ2 (Fig. 2 B, bottom traces, and D). The magnitude of peak ICa was nearly identical for all three groups of cells at different membrane potentials. In addition, the time course of ICa inactivation was similar at all test potentials. Taken together, these results show that CSQ2 represents a critical determinant of cardiac excitation–contraction coupling that controls the amount of Ca released from the SR by governing the duration of Ca release.
To gain further insight into the role of CSQ2 in cardiac excitation–contraction coupling, we performed measurements of elementary Ca-release events, i.e., Ca sparks (19), in control, CSQs, and CSQas myocytes that had been permeabilized by saponin. This approach allows application of membrane-impermeant agents and assessment of SR Ca release and storage properties under controlled experimental conditions (i.e., control over the composition of the cytosolic milieu) (20). The brightness of Ca sparks was increased significantly in CSQs myocytes and significantly reduced in CSQas myocytes compared with controls; however, the frequencies of these events were similar among all three groups (Fig. 3A). Differences in the magnitude and spatiotemporal spread of Ca sparks in the three groups are clearly evident in the averaged surface plots displayed in Fig. 3B. In addition, the duration of the rising phase of these local fluorescence signals but not their maximum rate of rise was affected by modulation of CSQ2 expression (Fig. 3 C and D). Specifically, when compared with control, CSQ2 overexpression resulted in ≈2-fold longer Ca spark rise times, whereas rise times were reduced to 80% in myocytes with reduced CSQ2 levels. The effects on distribution of rise times of individual events are documented in Fig. 3E. Therefore, the changes in Ca-release duration observed with measurements of cell-averaged Ca transients could be attributed, at least in part, to altered termination of elementary Ca-release events in myocytes with altered CSQ2 levels.
Fig. 3.
Effects of increased and reduced CSQ2 levels on properties of Ca sparks in permeabilized myocytes. (A) Representative line-scan images of Ca sparks acquired in myocytes infected with Ad-control, Ad-CSQs, and Ad-CSQas vectors. The average amplitudes (ΔF/F0) of sparks were 1.63 ± 0.01, 2.26 ± 0.03, and 1.32 ± 0.01 (n = 1,374–1,515); spatiotemporal frequencies were 4.79 ± 0.01, 4.76 ± 0.04, and 4.80 ± 0.02 events per second per 100 μm for control, CSQs, and CSQas cells, respectively. (B) Surface plots of averaged Ca sparks (3) in myocytes infected with Ad-control (42 events), Ad-CSQs (26 events), and Ad-CSQas (49 events) vectors. (C) Temporal profiles of averaged Ca sparks presented in A at the spatial peak. (Inset) Traces were scaled to the same peak amplitude. (D) The first derivative of the F/F0 traces shown in B.(E) Histograms of the rise times of individual Ca sparks in cells infected with Ad-control, Ad-CSQs, and Ad-CSQas vectors. The average rise times were 7.89 ± 0.01, 13.61 ± 0.02, and 6.32 ± 0.01 ms, for control, CSQs, and CSQas myocytes, respectively. All experiments were performed 48–56 h after infection of myocytes with the Ad constructs.
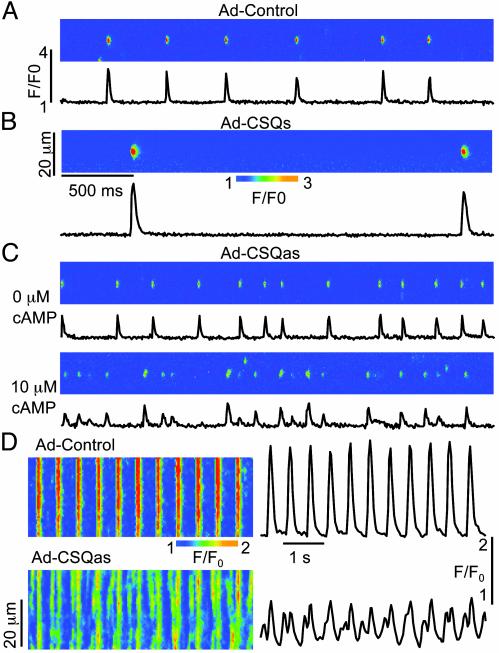
After Ca release, a certain amount of time must pass before Ca release can be triggered again. This refractoriness of the Ca release mechanism is not simply caused by the depletion of Ca from the SR but rather involves a desensitization of the Ca release channels to activating Ca (see ref. 32 for a review). To assess the impact of CSQ2 levels on the recovery of Ca release sites from this desensitized state, we measured repetitive Ca sparks induced at fixed locations by the high-affinity RyR activator imperatoxin A (21–23). In the presence of low doses (10 nM) of imperatoxin A, repeated events are apparently ignited by a single toxin-modified RyR that acts as a pacemaker for the whole release unit, and the frequency of events reflects the rate of functional recovery of Ca-release sites (3). In addition to increasing the frequency of transitions to the fully open state of the RyR, imperatoxin A also induces substates, which manifest themselves as long-lasting tails on Ca sparks (3, 21–23; unpublished results). To simplify interpretation of results, we excluded release sites exhibiting such long-lasting signals from our analysis (3). As shown in Fig. 4 A–C, repetitive Ca sparks were significantly less frequent in CSQs myocytes (0.57 ± 0.16 s–1, n = 92) and more frequent in CSQas myocytes (3.87 ± 0.09 s–1, n = 336), as compared with control (2.11 ± 0.07 s–1, n = 253). In addition, the magnitude of each spark was greater in CSQs myocytes and lower in CSQas myocytes. The frequency of sparks in CSQas cells was further enhanced by activation of endogenous protein kinase A (PKA) with cAMP (Fig. 4C Lower, 5.98 ± 0.10 s–1, n = 176). Stimulation of the PKA-mediated pathway accelerates the functional recovery of the Ca stores, apparently by stimulating Ca re-uptake (2). These results suggest that increased CSQ2 levels stabilize CICR by slowing the functional recovery of the Ca release sites from a refractory state, whereas reduced CSQ2 levels enhance the probability of Ca release from the SR by accelerating the RyR Ca-release channel recovery time.
Fig. 4.
Effects of altered CSQ2 levels on rhythmic Ca transients and restitution behavior of Ca-release sites. (A–C) Images of repetitive Ca sparks measured in the presence of 10 nM imperatoxin A in a control myocyte (A), a myocyte overexpressing CSQ2 (B), and a myocyte with reduced CSQ2 levels (C) in the absence (Upper) and in the presence (Lower) of 10 μM cAMP. All measurements were performed 48–56 h after infection with Ad-control, Ad-CSQs, and Ad-CSQas vectors. (D) Line-scan images along with averaged temporal profiles acquired in a control myocyte and in a myocyte with reduced CSQ2 levels stimulated at 2 Hz in the presence of 0.5 μM isoproterenol in the bathing solution.
Reduced CSQ2 protein levels due to mutations in the CSQ2 gene have been linked to polymorphic ventricular tachycardia and sudden death, which are induced by adrenergic compounds and exercise (16, 17). At the cellular level, various arrhythmias, including CPVT, are associated with spontaneous Ca release, causing delayed after-depolarization (24, 25). To test the hypothesis that abnormal restitution of SR Ca stores can lead to deregulation of Ca cycling in myocytes underexpressing CSQ2, we measured cytosolic Ca transients in control and CSQas myocytes undergoing periodic electrical stimulation. As shown in Fig. 4D, myocytes underexpressing CSQ2 exhibited arrhythmic Ca transients because of the generation of extrasystolic elevations of Ca; extra Ca transients were observed only on exposure to the β-adrenoreceptor agonist isoproterenol (0.5 μM; in four of five cells). No such disturbances in Ca cycling were observed in control myocytes in the presence of isoproterenol (n = 6). These findings are consistent with the role of adrenergic stimulation in determining clinical signs of CPVT, and suggest how reduced levels of CSQ2 might trigger arrhythmias in vivo.
Discussion
The present study has uncovered a role for the SR luminal Ca-binding protein, CSQ2 in regulating excitation–contraction coupling and CICR in the heart. Specifically, we show that Ad-mediated overexpression of CSQ2 dramatically increased the magnitude of both cell-averaged ICa-induced Ca transients and spontaneous Ca sparks in isolated heart cells. Analysis of the rising phase and rate of change of these Ca signals indicated that their augmented size was due to slowed termination of underlying Ca-release fluxes from the SR. In addition to prolonging active Ca release, the dynamics of functional recharging of SR Ca stores were also slowed in cells with elevated CSQ2 protein levels, as indicated by slowed recovery time for repetitive Ca sparks. We essentially obtained the opposite results in myocytes, in which CSQ2 levels were reduced by Ad-mediated antisense transduction. These myocytes displayed shorter Ca release but accelerated restitution of Ca-release sites. Furthermore, in periodically stimulated myocytes with decreased CSQ2 levels, application of isoproterenol caused arrhythmogenic oscillations of intracellular [Ca]. These results show that CSQ2 is a key determinant of the SR Ca releasing function that acts by governing Ca-release duration and release site refractoriness. In light of the observed isoproterenol -dependent disturbances in rhythmic Ca cycling in myocytes underexpressing CSQ2, our results are relevant to understanding the cellular mechanisms of catecholamine-induced arrhythmias linked to mutations of the CSQ2 gene.
Molecular Mechanisms of CSQ2 Action. We attribute the observed effects of CSQ2 on SR Ca release to the ability of this protein to function as a molecular buffer that controls the free [Ca] in the SR luminal space (see proposed scheme in Fig. 5). It is known from lipid bilayer studies that binding of Ca to the luminal aspect of the RyR complex (i.e., luminal Ca sensor) increases channel activity, whereas at lowered luminal [Ca], channel activity is reduced (EC50 ≈ 1 mM; ref. 26). Because many RyRs would be in an activated state at normal resting luminal Ca levels (≈1 mM), lowering luminal [Ca] during the release process would therefore decrease overall channel activity (a process termed luminal Ca-dependent deactivation; ref. 3). Our results suggest that by stabilizing the local free luminal [Ca] in the vicinity of RyRs during the release process, CSQ2 slows the luminal Ca-dependent closure of the RyR channel, thereby increasing the amount of Ca released from the SR to the cytosol. CSQ2 also controls the recovery dynamics of free intra-SR [Ca] during Ca re-uptake by serving as a sink for Ca in the SR lumen. Thus, CSQ2 not only potentiates the SR Ca efflux but also stabilizes CICR by modulating the functional restitution, or readiness, of Ca-release sites after each release. Recently, we invoked a similar mechanism to explain the effects of low-affinity Ca chelators (organic salts) loaded into the SR on Ca release (3). The importance of our present findings is that they show how the release process is controlled by an endogenous, SR-resident Ca-binding protein, CSQ2. They also reveal the pathologic consequences of reduced CSQ2 amounts in the SR of heart cells.
Fig. 5.
Illustration of the effects of increased and reduced CSQ2 levels on functional properties of the SR Ca store. The functional size of the SR Ca stores is determined by the level of CSQ2. The SR Ca-release channel is positively controlled by the free luminal [Ca] through the luminal Ca sensor. Increasing CSQ2 levels augments the amount of Ca released from SR by delaying the luminal [Ca]-dependent closure of the RyR channels. Reducing CSQ2 produces an opposite effect. After its discharge, the SR Ca store is refilled by SERCA. The store is functionally recharged when luminal Ca is reassociated with the luminal Ca sensor. Increased CSQ2 levels prolong the time required for functional recharging of the store, whereas reduced CSQ2 levels shorten the recovery. In CSQ2-underexpressing cells the RyRs are prone to premature activation. Increasing SERCA activity by adrenergic stimulation further accelerates recharging of the Ca store, providing an explanation for the role of catecholamines in triggering episodes of CPVT. JSR, junctional SR.
It has been proposed that CSQ2 could affect the Ca-release channel function through direct interactions with proteins comprising the Ca-release channel complex (i.e., RyR, junctin, and/or triadin) (ref. 13; for review, see ref. 9). Our studies have revealed no considerable differences in the parameters of SR Ca release between cells containing approximately 3-fold elevated levels of CSQ2 protein and cells in which organic buffers were loaded into the SR (3). Thus, the simplest explanation for our findings is that the predominant effects of CSQ2 on SR Ca release are due to the buffering actions of this protein inside the SR. Elucidation of the detailed mechanism by which CSQ2 and associated proteins such as junctin and triadin regulate Ca release must await studies involving manipulation of levels and expression of mutant forms of these proteins with altered abilities to interact among themselves and the RyR.
The maximal rate of Ca release from stimulated myocytes was similar regardless of the amounts of CSQ2 expressed in the SR (Figs. 2B and 3D). These results support the notion that CSQ2 controls termination of the release process, but these results are not what one would necessarily predict based on the expected buffering effects of this protein in the SR. Indeed, the maximal rate of release would be expected to be higher in cells with increased luminal Ca-buffering strengths caused by slowed decline of the free intra-SR [Ca], because, in these cells, the delayed decrease in the Ca gradient across the SR membrane would be expected to render larger Ca flux intensities. Several possible reasons explain why this was not observed. One possibility is that the initial free intra-SR [Ca] may be lower in CSQ2-overexpressing than in CSQ2-underexpressing cells. Although at steady state the free [Ca] in a closed compartment such as the SR should not be influenced by the number of Ca-binding sites (this should only influence the kinetics at which steady-state intra-SR [Ca] is attained), it is possible that a true steady state was not attained in our experiments (i.e., myocytes undergoing low rate stimulation). Another possibility is that the presence of elevated levels of CSQ2 protein slows the diffusion of Ca in the luminal space, thereby slowing the exodus of Ca through the open channels. Clearly more research, both experimental and theoretical, is needed to resolve this issue.
Termination of CICR. How CICR, a process with an inherent propensity to self-regeneration, is terminated has been a subject of intense research, first by using measurements of global Ca transients and, more recently, by examining Ca sparks (3, 27–30). One possibility that has been considered is that the RyR channels undergo Ca-dependent inactivation or adaptation as a result of the rise of [Ca] on the cytosolic side of the channel (27–29). Another possibility is that the decline in intra-SR [Ca] provides an active signal for closure of RyRs after they open (3, 30–32). We have recently found direct experimental evidence for a role of luminal Ca-induced changes in RyR activity (i.e., luminal Ca-dependent deactivation) in termination of SR Ca release by clamping the levels of intra-SR [Ca] with low-affinity Ca buffers loaded into the SR cisternae (3). Using these buffers, we dramatically prolonged the duration of active Ca release underlying both focal and global Ca transients in myocytes. Consistent with those findings, our present results show that increasing the levels of the high-capacity SR luminal Ca buffer CSQ2 prolongs Ca-release duration, whereas reducing amounts of this endogenous buffer shortened release duration, apparently by slowing or accelerating luminal Ca-dependent closure of RyRs, respectively. Together these results provide compelling evidence for the role of luminal Ca-dependent deactivation of RyRs in termination of CICR. Although demonstrating the importance of changes in luminal Ca in termination of SR Ca release, our results do not necessarily exclude the possibility that additional mechanisms, such as RyR inactivation or adaptation at cytosolic Ca regulatory sites might also influence Ca-release termination.
Although clear evidence exists for a role for changes in luminal Ca in the termination of cardiac sparks (ref. 3; present study), previous studies indicated that Ca sparks in skeletal muscle may not be accompanied by substantial depletion of the SR [Ca]. Lacampagne et al. (33) used kinetic analysis of high temporal resolution Ca spark recordings to suggest that the Ca-release flux underlying the Ca spark is constant in amphibian muscle fibers. Because SR Ca flux must decline whenever the SR [Ca] falls, this result could mean that luminal Ca levels remain steady during the Ca sparks. Thus, the possibility exists that the basic mechanisms responsible for spark termination are different in cardiac and skeletal muscle. The performance of complementary studies in these two cell types (i.e., modulation of CSQ protein levels in skeletal muscle fibers and rapid Ca spark kinetics measurements in cardiac myocytes) should help to clarify this issue.
Relevance to CPVT. One of the most important aspects of our results is that they show how reduced CSQ2 levels may cause cardiac arrhythmias at the cellular level. CPVT is an arrhythmogenic disease characterized by syncope and sudden death inducible by exercise and catecholamine infusion (34). Thus far, four mutations have been linked to CPVT. Three of these mutations appear to result in reduced CSQ2 expression (17), and one mutation has been proposed to lead to disrupted binding of Ca (16). Our results suggest that the underlying cause of CPVT might be related to abnormal restitution of the Ca-release mechanism caused by reduced CSQ2 levels (Fig. 5). Because of the lower concentration of Ca-binding sites in the SR of cells underexpressing CSQ2, refilling of the Ca store by the SERCA pump occurs faster than in normal cells. Store recharging becomes even faster when the activity of SERCA is stimulated by protein kinase A, potentially accounting for the dependence of clinical episodes of CPVT on catecholamines. In myocytes with decreased CSQ2 levels, premature recovery of Ca-release channels from a luminal Ca-dependent refractory state led to spontaneous, extrasystolic discharges of SR Ca stores. It is known that spontaneous Ca release can induce inward currents and oscillations in the membrane potential, resulting in triggered arrhythmia (4–7). Therefore, the observed disturbances in Ca handling could explain, at least in part, the pathogenesis of CPVT associated with genetic defects that result in either reduced Ca-binding activity (16) or reduced expression levels of CSQ2 (17).
Comparison with Previous Studies in Transgenic Mice. Our findings differ from previous results obtained in transgenic mice overexpressing CSQ2 (14, 15). In those studies, a 10- to 20-fold overexpression of CSQ2 increased SR Ca storage capacity of isolated cardiac myocytes; however, the luminal Ca was not available for release, leading to depressed global and local Ca-release signals. These changes in Ca handling were accompanied by several structural, functional, and biochemical alterations at the cellular and subcellular levels and development of cardiac hypertrophy and failure. On the other hand, the present study maintains CSQ2 protein levels closer to physiological levels under acute rather than chronic conditions; thus, these data are likely to more accurately reflect the direct physiological consequences of changes in CSQ2 protein levels on intracellular Ca handling. These results highlight the potential problems in interpreting the effects of constitutive high-level expression of proteins in transgenic animal models and the value of complementary studies in more acute settings.
Conclusion. In conclusion, we have found that CSQ2 is an essential determinant of the ability of the SR to store and release Ca in cardiac muscle. CSQ2 appears to serve as a reservoir for Ca that is readily accessible for CICR and also as an active Ca buffer that modulates local luminal Ca-dependent closure of RyRs. At the same time, CSQ2 stabilizes the CICR mechanism by slowing the functional recharging of SR Ca stores. Abnormal repriming behavior of Ca-release channels could account for, or contribute to, ventricular arrhythmias associated with mutations in the CSQ2 gene.
Acknowledgments
We thank Dr. L. R. Jones for providing cDNA for CSQ2 and Dr. H. H. Valdivia for providing imperatoxin A. This work was supported by American Heart Association Grants 0250047N (to S.G.) and 0245088N (to S.C.W.) and National Institutes of Health Grant HL03739 (to S.G.). S.C.W. is a Neely–Treadwell Cancer Investigator.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: Ad, adenovirus; RyR, ryanodine receptor; SR, sarcoplasmic reticulum; CSQ, calsequestrin; CSQ2, cardiac CSQ; CPVT, catecholaminergic polymorphic ventricular tachycardia; CICR, calcium-induced calcium release.
References
- 1.Fabiato, A. (1985) J. Gen. Physiol. 85, 247–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bers, D. M. (2002) Nature 415, 198–205. [DOI] [PubMed] [Google Scholar]
- 3.Terentyev, D., Viatchenko-Karpinski, S., Valdivia, H. H., Escobar, A. L. & Györke, S. (2002) Circ. Res. 91, 414–420. [DOI] [PubMed] [Google Scholar]
- 4.Kass, R. S. & Tsien, R. W. (1982) Biophys. J. 38, 259–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Allen, D. G., Eisner, D. A. & Orchard, C. H. (1984) J. Physiol. 352, 113–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lakatta, E. G. & Guarnieri, T. (1993) J. Cardiovasc. Electrophysiol. 4, 473–489. [DOI] [PubMed] [Google Scholar]
- 7.Marban, E., Robinson, S. W. & Wier, W. G. (1986) J. Clin. Invest. 78, 1185–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yano, K. & Zarain-Herzberg, A. (1994) Mol. Cell. Biochem. 135, 61–70. [DOI] [PubMed] [Google Scholar]
- 9.Györke, S., Györke, I., Lukyanenko, V., Terentyev, D., Viatchenko-Karpinski, S. & Wiesner, T. F. (2002) Front. Biosci. 7, d1454–d1463. [DOI] [PubMed] [Google Scholar]
- 10.Mitchell, R. D., Simmerman, H. K. & Jones, L. R. (1983) J. Biol. Chem. 263, 1376–1381. [PubMed] [Google Scholar]
- 11.Gala, S. E. & Jones, L. R. (1983) J. Biol. Chem. 258, 11932–11936. [PubMed] [Google Scholar]
- 12.Guo, W., Jorgensen, A. O., Jones, L. R. & Campbell, K. P. (1996) J. Biol. Chem. 271, 458–465. [DOI] [PubMed] [Google Scholar]
- 13.Zhang, L., Kelley, J., Schmeisser, G., Kobayashi, Y. M. & Jones, L. R. (1997) J. Biol. Chem. 272, 23389–23397. [DOI] [PubMed] [Google Scholar]
- 14.Sato, Y., Ferguson, D. G., Sako, H., Dorn, G. W., II, Kadambi, V. J., Yatani, A., Hoit, B. D., Walsh, R. A. & Kranias, E. G. (1998) J. Biol. Chem. 273, 28470–28477. [DOI] [PubMed] [Google Scholar]
- 15.Wang, W., Cleemann, L., Jones, L. R. & Morad, M. (2000) J. Physiol. 524, 399–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lahat, H., Pras, E., Olender, T., Avidan, N., Ben-Asher, E., Man, O., Levy-Nissenbaum, E., Khoury, A., Lorber, A., Goldman, B., et al. (2001) Am. J. Hum. Genet. 69, 1378–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Postma, A. V., Denjoy, I., Hoorntje, T. M., Lupoglazoff, J. M., Da Costa, A., Sebillon, P., Mannens, M. M., Wilde, A. A. & Guicheney, P. (2002) Circ. Res. 91, e21–e26. [DOI] [PubMed] [Google Scholar]
- 18.Scott, B. T., Simmerman, H. K., Collins, J. H., Nadal-Ginard, B. & Jones, L. R. (1988) J. Biol. Chem. 263, 8958–8964. [PubMed] [Google Scholar]
- 19.Cheng, H., Lederer, W. & Cannell, M. B. (1993) Science 262, 740–744. [DOI] [PubMed] [Google Scholar]
- 20.Lukyanenko, V. & Györke, S. (1999) J. Physiol. 521, 575–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tripathy, A., Resch, W., Xu, L., Valdivia, H. H. & Meissner, G. (1998) J. Gen. Physiol. 111, 679–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gonzalez, A., Kirsch, W. G., Shirokova, N., Pizarro, G., Brum, G., Pessah, I. N., Stern, M. D., Cheng, H. & Rios, E. (2000) Proc. Natl. Acad. Sci. USA 97, 4380–4385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shtifman, A., Ward, C. W., Wang, J., Valdivia, H. H. & Schneider, M. F. (2000) Biophys. J. 79, 814–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lakatta, E. G. (1992) Cardiovasc. Res. 26, 193–214. [DOI] [PubMed] [Google Scholar]
- 25.Priori, S. G. & Corr, P. B. (1990) Am. J. Physiol. 258, H1796–H1805. [DOI] [PubMed] [Google Scholar]
- 26.Györke, I. & Györke, S. (1998) Biophys. J. 75, 2801–2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yasui, K., Palade, P. & Györke, S. (1994) Biophys. J. 67, 457–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lukyanenko, V., Wiesner, T. F. & Györke, S. (1998) J. Physiol. 507, 667–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sham, J. S., Song, L. S., Chen, Y., Deng, L. H., Stern, M. D., Lakatta, E. G. & Cheng, H. (1998) Proc. Natl. Acad. Sci. USA 95, 15096–15101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.DelPrincipe, F., Egger, M. & Niggli, E. (1999) Nat. Cell Biol. 1, 323–329. [DOI] [PubMed] [Google Scholar]
- 31.Lukyanenko, V., Györke, I. & Györke, S. (1996) Pflügers Arch. 432, 1047–1054. [DOI] [PubMed] [Google Scholar]
- 32.Sobie, E. A., Dilly, K. W., Cruz, J. D., Lederer, W. J. & Jafri, M. S. (2002) Biophys. J. 83, 59–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lacampagne, A., Ward, C. W., Klein, M. G. & Schneider, M. F. (1999) J. Gen. Physiol. 113, 187–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leenhardt, A., Lucet, V., Denjoy, I., Grau, F., Ngoc, D. D. & Coumel, P. (1995) Circulation 91, 1512–1519. [DOI] [PubMed] [Google Scholar]