Abstract
SOS2 (salt overly sensitive 2) is a serine/threonine protein kinase required for salt tolerance in Arabidopsis thaliana. In this study, we identified the protein phosphatase 2C ABI2 (abscisic acid-insensitive 2) as a SOS2-interacting protein. Deletion analysis led to the discovery of a novel protein domain of 37 amino acid residues, designated as the protein phosphatase interaction (PPI) motif, of SOS2 that is necessary and sufficient for interaction with ABI2. The PPI motif is conserved in protein kinases of the SOS2 family (i.e., protein kinase S, PKS) and in the DNA damage repair and replication block checkpoint kinase, Chk1, from various organisms including humans. Mutations in the conserved amino acid residues in the PPI motif abolish the interaction of SOS2 with ABI2. We also identified a protein kinase interaction domain in ABI2 and examined the interaction specificity between PKS and the ABI phosphatases. We found that some PKSs interact strongly with ABI2 whereas others interact preferentially with ABI1. The interaction between SOS2 and ABI2 was disrupted by the abi2-1 mutation, which causes increased tolerance to salt shock and abscisic acid insensitivity in plants. Our results establish the PPI motif and the protein kinase interaction domain as novel protein interaction domains that mediate the binding between the SOS2 family of protein kinases and the ABI1/2 family of protein phosphatases.
Reversible protein phosphorylation is a fundamental mechanism by which living organisms regulate cellular processes in response to developmental, hormonal, and environmental cues. The Arabidopsis SOS2 (salt overly sensitive 2) gene is necessary for sodium and potassium ion homeostasis and salt tolerance (1). SOS2 encodes a serine/threonine protein kinase with an N-terminal kinase catalytic domain similar to SNF1/AMPK and a novel C-terminal regulatory domain (2). SOS2 is normally inactive, presumably because of an intramolecular interaction between the catalytic domain and the autoinhibitory regulatory domain (3). Salt stress elicits a cytosolic calcium signal (4). Calcium, together with the calcium-binding protein SOS3, activates SOS2 (5). SOS3 physically interacts with SOS2 in the yeast two-hybrid system as well as in vitro (5). A 21-aa sequence in the regulatory domain of SOS2, designated as the FISL motif, is necessary and sufficient for the interaction with SOS3 (3). The SOS3–SOS2 kinase complex is required for the phosphorylation and activation of the plasma membrane Na+/H+ antiporter encoded by the SOS1 gene (6–8).
In Arabidopsis, SOS2 is a member of a family of 25 protein kinases that are known as protein kinase S (PKS) (3). Evidence suggests that individual PKS proteins interact with specific calcium-binding proteins in the SOS3 family (known as SCaBPs) to form distinct protein kinase complexes (3). These protein kinase complexes may be capable of decoding various calcium signals elicited by developmental, hormonal, or environmental cues (3). For example, whereas the SOS3–SOS2 protein kinase complex mediates salt stress-specific calcium signaling, SCaBP5 and PKS3 form a complex that participates in a regulatory loop in abscisic acid (ABA) signaling, possibly by decoding an ABA-elicited calcium signal and by controlling the generation of such a calcium signal (9).
The phosphorylation status of a protein is determined by the balance between the activities of protein kinases and protein phosphatases. Protein phosphatase 2C is a class of conserved serine/threonine protein phosphatases involved in stress responses in plants, yeasts, and animals (10–13). Dominant mutations in two of the homologous protein phosphatase 2Cs, ABA-insensitive (ABI) 1 and ABI2, render Arabidopsis plants insensitive to the stress hormone ABA (8, 14–18). The abi1-1 and abi2-1 mutations have been proposed to have dominant negative effects, and the WT ABI1 and ABI2 proteins are thought to be negative regulators of ABA signaling (19–21). Although ABI1 and ABI2 are highly homologous, they may function at different steps in ABA signaling (22–26).
In this study, we identified ABI2 as a SOS2-interacting protein. Deletion analysis led to the discovery of a novel protein domain of 37 amino acid residues, designated as the protein phosphatase interaction (PPI) motif, of SOS2 that is necessary and sufficient for interaction with ABI2. The PPI motif is conserved in Arabidopsis PKS proteins and in the DNA damage repair and replication block checkpoint kinase, Chk1, from various organisms including humans. Mutations in the conserved amino acid residues in the PPI motif abolish the interaction of SOS2 with ABI2. We also identified a protein kinase interaction (PKI) domain in ABI2 and examined the interaction specificity between PKS and the ABI phosphatases. We found that some PKSs interact strongly with ABI2 whereas others interact preferentially with ABI1. The interaction between SOS2 and ABI2 was disrupted by the abi2-1 mutation, which causes increased tolerance to salt shock and ABA insensitivity in plants. Our identification of the PPI motif and the PKI domain that mediate the interaction between SOS2 and ABI2 contributes substantially to the understanding of the structure and regulation of these key regulators of stress tolerance.
Materials and Methods
Construction of Plasmids. Plasmids pAS-SOS2 and pAS-SOS2N were described by Halfter et al. (5) and Guo et al. (3), respectively. Plasmids pAS-SOS1, pACT2-ABI1, pact-ABI2, and pASPKS3 were constructed previously (9). Plasmids containing the C-terminal portions of SOS2 cDNA (pAS-SOS2-T1 and pASSOS2-T3) were constructed by moving the NcoI and BamHI fragments from pACT-SOS2-T1 and pACT-SOS2-T3 (3) into the pAS2 vector. Full-length cDNAs for PKS11, PKS18, and PKS24 were amplified by PCR using specific primers and inserted into the pAS2 vector to make pAS-PKS11, pAS-PKS18, and pAS-PKS24. The pACT2-SOS3 plasmid was made by moving a SmaI and an EcoRI fragment from pGEX-SOS3 (5) to pACT2.
To make pAS-SOS2 and pAS-PKS3 deletion constructs [pASSOS2 (333/385), pAS-SOS2 (333/369), pAS-SOS2 (386/446), and pAS-PKS3 (327/371)], PCR was performed with pairs of forward primers (containing an NcoI site) and reverse primers (containing a BamHI site). The resulting PCR products were digested with NcoI and BamHI and inserted between the NcoI and BamHI sites of pAS2. Deletion constructs of pACT-ABI2 (1/224, 1/111, 112/224, 225/423, 112/147, and 148/193) were made by PCR amplification using specific primers and inserting the DNA fragments into the SmaI and EcoRI sites of pACT2.
Inverse PCR-based site-directed mutagenesis was carried out on double-stranded plasmid DNA with high-fidelity Taq polymerase by using the following primers: 5′-AGAAACAGCAGCGGTTTGCCTTTTAAC-3′ and 5′-CGAAGGGAACCTAGTGAG-3′ for pAS-SOS2 (R340A/F341A); 5′-TTGTGATAAGGTAATCATC-3′ and 5′-GGCTTAAATGACTCTGCAC-3′ for pAS-SOS2 (L324D); 5′-GCCATCGTAAACATCAAAG-3′ and 5′-CATGGCGGTTCTCAGG-3′ for pACT-ABI2 (G162D); 5′-GCCATCGTAAACACCAAAG-3′ and 5′-CATGACGGTTCTCAGG-3′ for pACT-ABI2 (G168D); 5′-CTCCTTCGTCAAAGCCAG-3′ and 5′-ATAGTGAAGGAGAAACCG-3′ for pACT-ABI2 (E186K); 5′-CTCCGTCAAAGCCAAATG-3′ and 5′-GAGATAGCTA AGGAGA A AC-3′ for pACT-A BI1 (A197T); 5′-CTCCGCCA A AGCCA A ATG-3′ and 5′-GAGATAGTTAAGGAGAAAC-3′ for pACT-ABI1 (A201V); and 5′-CTCCGTCAAAGCCAAATG-3′ and 5′-GAGATAGTTAAGGAGAAAC-3′ for pACT-ABI1 (A197TA201V). The resulting PCR products were digested with DpnI and purified by gel electrophoresis, followed by self-ligation. The ligated products were transformed into Escherichia coli DH5a cells. All plasmid constructs were completely sequenced to ensure that there were no PCR or cloning errors.
Yeast Two-Hybrid Screen and Interaction Assays. The SOS2 coding region was amplified by PCR with primers containing restriction sites and cloned in-frame between the NcoI and BamHI sites of pAS2 to make the bait plasmid pAS-SOS2. The screening of pACT plasmid library (27) was performed as described (5).
Plasmid DNA of bait and prey constructs were transformed into the Saccharomyces cerevisiae strain Y190. The transformants were grown overnight at 30°C in synthetic complete (SC) media lacking tryptophan, uracil, and leucine. Twenty microliters of the cell suspension containing ≈4 × 104 cells was dropped onto SC agar plates lacking tryptophan, uracil, and leucine, and the cells were grown for 2 days at 30°C. After colonies were transferred onto a nitrocellulose transfer membrane (NT, BioTrace, 0.45 mm; Gelman), β-galactosidase (β-gal) filter assays were carried out as described (5).
In Vitro Protein Binding Assays. To produce E. coli-expressed GST-ABI2, the coding region of ABI2 was cloned in-frame into the BamHI and EcoRI sites of pGEX-2TK. The resulting construct was transformed into E. coli BL21 DE3 cells to obtain the GST-ABI2 fusion protein. The 37-aa PPI sequence of SOS2 was cloned into the BamHI and EcoRI sites of pGEX-2TK to produce the GST-PPI fusion protein. Radiolabeled ABI2 and SOS2 were produced from pET14b-ABI2 and pET14b-SOS2, respectively, using an in vitro transcription and translation assay kit (TNT Coupled Reticulocyte Lysate System, Promega) with [35S]methionine as the sole source of methionine, according to the manufacturer's instructions. In vitro protein affinity pull-down assays were carried out as described (3).
Plant Growth and Salt Treatments. Seeds of WT and mutants were sown on Murashige and Skoog (MS) plates containing 1.2% (wt/vol) agar and 3% sucrose. The seeds were stratified at 4°C for 3 days and then transferred to 22°C under continuous light for germination and growth. Five days after germination, seedlings of WT and mutants were transferred onto either MS agar plates or MS agar plates containing 150 mM NaCl for salt shock treatments.
Results
Identification of ABI2 as a SOS2-Interacting Protein. Using a yeast two-hybrid approach, we screened for proteins that interact with the protein kinase SOS2. The entire SOS2 cDNA was fused in-frame with the DNA binding domain of GAL4 to generate a bait construct in the plasmid pAS2, and the construct was transformed into the yeast strain Y190 (28). We screened a λ-ACT cDNA library prepared from mRNA isolated from young Arabidopsis seedlings (27). Sequence analysis revealed that 7 of the 101 putative interacting clones that we isolated encode ABI2 (see refs. 17 and 18).
A combination of the bait plasmid pAS-SOS2 with the empty prey vector pACT2 did not activate transcription of the β-gal reporter gene (Fig. 1A). Strong β-gal activity was observed when the SOS2 bait was combined with ABI2 in the prey vector (Fig. 1 A). To confirm whether SOS2 can bind to ABI2 in vitro, an affinity pull-down assay was carried out. [35S]Methioninelabeled SOS2 protein was incubated with the GST-ABI2 or GST-RB (maize retinoblastoma protein; see ref. 5) fusion protein on Sepharose beads. The beads were pelleted and washed, and the bound proteins were resolved by SDS/PAGE. The labeled SOS2 protein was detected from the GST-ABI2 beads but not from the control GST-RB beads, demonstrating that SOS2 can bind to ABI2 in vitro (Fig. 1B).
Fig. 1.
SOS2 interacts with ABI2. (A) SOS2 interacts strongly with ABI2 but only weakly with ABI1 in the yeast two-hybrid assay. The pAS-SOS2/pACT2, pAS-SOS1/pACT-ABI1, and pAS-SOS1/pACT-ABI2 combinations were used as negative controls. Yeast strains containing the pAS-SOS2 or pAS-SOS1 bait and the pACT-ABI1 or pACT-ABI2 prey were assayed for β-gal activity (blue). Yeast grown on SC plates (Left) and the corresponding β-gal filter assays (Right) are shown. (B) SOS2 binds to ABI2 in vitro. [35S]Methionine-labeled SOS2 was pulled down by GST-ABI2 but not by GST-RB. GST-RB was used as a negative control.
Although ABI1 is very similar in sequence to ABI2 (15, 16), we did not find ABI1 among the putative interacting clones from the yeast two-hybrid screen (data not shown). A combination of the SOS2 bait and ABI1 in the prey vector led only to a slight activation of the β-gal reporter gene expression (Fig. 1 A). As a negative control, a combination of the C-terminal tail of SOS1 (6) as a bait with either ABI1 or ABI2 in the prey vector failed to activate the reporter gene (Fig. 1 A). Together, these results show that SOS2 preferentially binds to ABI2.
Identification of an ABI2-Binding Motif in SOS2. To identify a minimal region of SOS2 that is sufficient for interaction with ABI2, we made serial deletions of SOS2 in the bait vector pAS2 (Fig. 2A). When combined with the empty prey plasmid pACT2, none of the deletion constructs of SOS2 baits activated the β-gal reporter gene (Fig. 2B). The bait construct SOS2-T1, which contains the C-terminal regulatory domain of SOS2, activated transcription of the β-gal reporter gene when combined with the ABI2 prey plasmid (Fig. 2B). In contrast, the N-terminal region of SOS2 (SOS2-N), which corresponds to the kinase catalytic domain (2, 3, 5), did not activate the reporter gene. These results indicate that the C-terminal, but not the N-terminal, region of SOS2 interacts with ABI2.
Fig. 2.
Identification of a PPI motif in SOS2 by deletion mapping. (A) Schematic representation of the constructs used in the yeast two-hybrid interaction assay. The indicated regions of SOS2 were cloned into the bait vector pAS2. (B) Interaction in the yeast two-hybrid assay. The SOS2 bait plasmids were transformed with either pACT2 or pACT-ABI2 into the yeast strain Y190. Yeast grown on SC plates (Upper) and β-gal filter assay (Lower) are shown. (C) In vitro binding assay. [35S]Methionine-labeled ABI2 protein was pulled down by GST-PPI but not by GST.
The FISL motif (amino acids 309–329) of SOS2 mediates the interaction with SOS3 (3). SOS2-T3, which does not contain the FISL motif, also interacted with ABI2 (Fig. 2B), showing that the FISL motif is not important for SOS2 interaction with ABI2. We found that the SOS2 sequence between amino acid residues 333 and 385 but not 386 and 446 interacted with ABI2 (Fig. 2B). Further analysis showed that the SOS2 sequence between amino acids 333 and 369 is sufficient for interaction with ABI2 in the yeast two-hybrid system (Fig. 2B). We designated this 37-aa sequence of SOS2 the PPI motif.
To determine whether the 37-aa PPI motif can bind ABI2 in vitro, we carried out a pull-down assay with 35S-labeled ABI2 and GST-PPI fusion protein or GST alone on Sepharose beads. ABI2 was found to bind to the GST-PPI but not GST control beads (Fig. 2C). The result shows that the PPI peptide is sufficient for binding to ABI2 in vitro.
The PPI Motif Is Conserved in PKS. Previously, we showed that a SOS2-like protein kinase, PKS3, interacts strongly with ABI2 and weakly with ABI1 (9). To examine whether other PKSs can interact with ABI1 and/or ABI2, we performed yeast two-hybrid assays with PKS11 (29), PKS18 (30), and PKS24 (3) as baits. PKS11 and PKS24 interacted preferentially with ABI2 but not with ABI1 (Fig. 3A). In contrast, PKS18 interacted strongly with ABI1 but only weakly with ABI2 (Fig. 3A).
Fig. 3.
Differential interaction between PKS and ABI proteins mediated by the PPI motif. (A) Interaction of PKS11, PKS18, and PKS24 with ABI1 and ABI2. Yeast strains containing the pAS-PKS11, pAS-PKS18, and pAS-PKS24 baits and pACT-ABI1 and pACT-ABI2 prey were assayed for β-gal activity. Combinations with the empty pACT2 prey vector were used as negative controls. Yeast grown on SC plates (Left) and β-gal filter assay (Right) are shown. (B) Alignment of the PPI sequences of SOS2 and PKS proteins. A putative regulatory region of the human protein kinase Chk1 is shown under the alignment. Identical and similar amino acid residues are shaded in black and gray, respectively. Asterisks indicate amino acid residues identical between Chk1 and at least two of the plant proteins. (C) The PPI motif in PKS3 is sufficient for interaction with ABI2. Full-length PKS3 or its PPI motif (amino acids 327–371) in bait vector was transformed with either pACT2 or pACT-ABI2 into the yeast strain Y190 for two-hybrid assay. Yeast grown on SC plates (Left) and β-gal filter assay (Right) are shown.
Sequence alignment shows that the PPI motif of SOS2 is conserved in these protein kinases (Fig. 3B). Interestingly, the motif is also conserved in the DNA replication checkpoint kinase Chk1 from humans (Fig. 3B). To determine whether the putative PPI motif of PKS3 can mediate its interaction with ABI2, we made a bait plasmid containing amino acids 327–371 of PKS3. As shown in Fig. 3C, this putative PPI sequence interacted with ABI2.
In the PPI motif of SOS2, Arg-340 and Phe-341 are highly conserved among the protein kinases (Fig. 3B). To further elucidate the amino acids important for the kinases to interact with protein phosphatases, we mutated these two residues of SOS2 and tested the impact of the mutations on the protein interaction. Interaction of the SOS2 bait with the ABI2 prey was abolished when Arg-340 and Phe-341 were both substituted with alanine (R340AF341A) (Fig. 4), suggesting that these two conserved residues in the protein kinase are important for interaction with the ABI phosphatases. In comparison, substitution of Leu-324 with aspartic acid (L324D), which is located within the FISL motif, did not affect the interaction of SOS2 with ABI2 (Fig. 4B). As expected, the L324D mutation abolished the interaction with SOS3 (Fig. 4B). The R340AF341A mutations also weakened the interaction with SOS3 (Fig. 4B), which may be caused by a steric hindrance effect of the mutations on the neighboring FISL motif.
Fig. 4.
Mutational analysis of the PPI motif in SOS2. (A) The WT sequence of SOS2 spanning the FISL motif and the PPI motif is shown. Arrows indicate introduced amino acid substitutions. (B) Interaction in the yeast two-hybrid system. WT and the mutated SOS2-bait plasmids were transformed with pACT2, pACT-ABI2, or pACT-SOS3 into the yeast strain Y190. Yeast grown on SC plates (Left) and β-gal filter assay (Right) are shown.
Identification of a SOS2-Interacting Domain in ABI2. To determine which region of ABI2 is involved in the interaction with SOS2, we made deletion constructs of ABI2 in the prey plasmid pACT2 (Fig. 5A), and their interaction with SOS2 was evaluated by using the yeast two-hybrid assay. The N-terminal region (amino acids 1–224, 1/224) of ABI2 retained the interaction with SOS2, whereas the C-terminal region (amino acids 225–423, 225/423) did not interact with SOS2 (Fig. 5B). This finding suggests that the N-terminal half of ABI2 is sufficient for the interaction with SOS2, although the interaction is not as strong as for the full-length ABI2. Weak but significant β-gal activity was still observed when the prey plasmid harbored only amino acid residues 112–224 (112/224) of ABI2 (Fig. 5B). Further deletions narrowed the SOS2 interaction domain of ABI2 to a 46-aa region (amino acids 148–193) (Fig. 5B).
Fig. 5.
Identification of a SOS2 interaction domain in ABI2. (A) Schematic representation of the constructs used in the yeast two-hybrid interaction assay. The indicated regions of ABI2 were cloned into the prey vector pACT2. (B) Interaction in the yeast two-hybrid assay. The pAS-SOS2 bait was transformed with the ABI2 prey plasmids into the yeast strain Y190. Yeast grown on SC plates (Left) and β-gal filter assay (Right) are shown.
The abi2-1 Mutant Protein Cannot Interact with SOS2. The PKI domain of ABI2 is conserved among ABI1, ABI2, and other protein phosphatase 2Cs from Arabidopsis (Fig. 6A). Several amino acid residues in this region are known to be important for the function of ABI1 and ABI2. In the dominant abi2-1 mutant of Arabidopsis, Gly-168 is substituted by an aspartic acid residue (17, 18). Substitution of amino acid Gly-174 of ABI1 by aspartic acid, which corresponds to Gly-162 of ABI2, abolishes the ability of ABI1 to block ABA-inducible transcription in maize protoplasts (19). A recessive mutation (R1) in the abi2-1 mutant background, which converts Glu-186 to lysine, suppresses the ABI phenotype of the abi2-1 mutant (21). To determine whether these mutations might affect the interaction of ABI2 with SOS2, these mutations (G162D, G168D, and E186K) were introduced into the ABI2 prey construct, and the interaction of the resultant ABI2 mutant proteins with SOS2 was evaluated by the yeast two-hybrid assay. Either of the G162D and G168D mutations in ABI2 abolished the interaction with SOS2 (Fig. 6B). However, the E186K mutation in ABI2 did not prevent the interaction with SOS2 (Fig. 6B). These results further confirmed that some of the amino acid residues in the SOS2 interaction domain (amino acids 148–193) are important for ABI2 to interact with SOS2.
Fig. 6.
Mutational analysis of the PKI domains of ABI1 and ABI2. (A) Alignment of several Arabidopsis protein phosphatase 2Cs in the PKI domain. Identical and conserved amino acid residues are shaded in black and gray, respectively. Arrows indicate positions of amino acid substitutions. (B) Mutational analysis of the PKI domain in ABI2. Yeast strains containing the pASSOS2 bait combined with WT or the mutated ABI2 in the pACT2 prey vector were assayed for β-gal activity. Yeast grown on SC plates (Left) and β-gal filter assay (Right) are shown. (C) Mutational analysis of the PKI domain in ABI1. Yeast strains containing the pAS-SOS2 bait combined with WT or the mutated ABI1 in the pACT2 prey vector were assayed for β-gal activity. pACT2 and pACT-ABI2 were used as negative and positive controls, respectively. Yeast grown on SC plates (Left) and β-gal filter assay (Right) are shown.
Amino Acid Substitutions in ABI1 That Increase Its Interaction with SOS2. The minimal SOS2 interaction domain of ABI2 is highly conserved in ABI1. In particular, amino acid residues 158–193 of ABI2 are almost identical to those of ABI1 except that the Thr-197 and Val-201 in ABI2 are replaced by Ala in ABI1 (Fig. 6A). Despite this highly conserved sequence, ABI1 interacts only weakly with SOS2 (Figs. 1 A and 6C). To test whether the two divergent amino acid residues (Thr-197 and Val-201 in ABI2) contribute to the specificity of the interaction with SOS2, we introduced targeted mutations that convert the amino acid residues of ABI1 into those of ABI2. Single amino acid substitutions in ABI1 (A197T and A201V) did not result in stronger interactions with SOS2 (Fig. 6C). However, a double amino acid substitution in ABI1 (A197TA201V) conferred a stronger interaction with SOS2 (Fig. 6C). These results suggest that the two divergent amino acid residues are important for SOS2 to distinguish ABI2 from ABI1.
The abi2-1 Mutant Is Tolerant to Salt Shock. The protein kinase SOS2 is a positive regulator of salt tolerance, and sos2 mutants are hypersensitive to salt stress (1). To begin to determine the in vivo role of ABI2 in the SOS regulatory pathway for salt tolerance, we examined the salt tolerance of the abi1-1 and abi2-1 mutants (Fig. 7). WT, abi1-1, and abi2-1 plants were germinated and grown on normal MS agar medium without NaCl for 5 days and then transferred onto MS agar medium either with or without supplementation of 150 mM NaCl. Without NaCl supplementation, the abi1-1 and abi2-1 mutants grew as well as the WT. However, in the presence of 150 mM NaCl for 6 days, the WT seedlings were killed, whereas the abi1-1 and abi2-1 plants were still alive. Therefore, the abi1-1 and abi2-1 mutants are more tolerant to salt shock than the WT plants. This result suggests that ABI2 is involved in salt tolerance in vivo.
Fig. 7.
abi1-1 and abi2-1 mutant seedlings are more tolerant to salt shock. WT and the abi1-1 and abi2-1 mutant seedlings were grown on vertical MS plates containing 1.2% (wt/vol) agar and 3% sucrose. After 5 days of germination, the seedlings were transferred onto either the control MS agar plates (Left) or MS agar plates containing 150 mM NaCl (Right). The pictures were taken 6 days after the seedling transfer.
Discussion
Protein–protein interactions are fundamental to our understanding of protein regulation and signal transfer mechanisms of signal transduction. The interactions between proteins are often mediated by discrete domains and even by short sequence motifs. Well-known protein interaction domains include those designated SH3, SH2, WW, EH, and PDZ (31, 32). Undoubtedly, the identification of protein interaction domains is a critical part of signal transduction studies and contributes to a fundamental knowledge base of cell biology (31, 32). However, very few protein interaction domains have been defined in plant proteins.
SOS2 is a serine/threonine protein kinase that is required for plant salt tolerance (2). We have shown that SOS2 interacts with and is activated by SOS3 (5). SOS3 binds to the FISL motif, a 21-aa sequence close to the kinase catalytic domain of SOS2 (3). In this study, we show that SOS2 also interacts with the protein phosphatase 2C ABI2 and identify a minimal ABI2 binding sequence in SOS2. This sequence is designated as the PPI motif and is adjacent to the FISL motif. The identification of this PPI motif represents an important contribution to our understanding of the structure and regulation of SOS2.
The PPI motif is conserved in the SOS2 class of protein kinases, i.e., PKS. Indeed, of several PKS proteins tested, all were found to interact with ABI2 and/or ABI1. The PPI motif in PKS3 is also sufficient for interaction with ABI2. PKS represent a large family of 25 protein kinases (3), and there are eight 2C-type protein phosphatases in the ABI2/ABI1 subclass (33). Sequence variations in the PPI motif of PKS may determine whether a particular PKS interacts with ABI2, ABI1, or another related protein phosphatase. For example, residues R340 and S343 of SOS2 are conserved in PKS3, PKS11, and PKS18, all of which interact strongly with ABI2 but not ABI1 (Fig. 3B). In contrast, these two residues are changed to K and T in PKS18, which likely explains why PKS18 interacts strongly with ABI1 but only weakly with ABI2. On the protein phosphatase side, variations in the PKI domain likely determine which protein phosphatase interacts with which PKS. Indeed, although the WT ABI1 protein interacts only weakly with SOS2, substitutions of two amino acid residues within the PKI domain with corresponding residues in ABI2 led to an interaction with SOS2 that was nearly as strong as that of ABI2.
The G162D and G168D mutations in the PKI domain disrupt the interaction between ABI2 and SOS2. These mutations also have important functional consequences on ABA signaling and salt tolerance. In fact, the G168D mutation is responsible for the dominant abi2-1 mutant phenotypes in ABA insensitivity (17) and tolerance to salt shock. The G162D mutation in ABI2 corresponds to the G174D mutation in ABI1, which has been shown to abolish the ability of ABI1 to block ABA-inducible transcription in maize protoplasts (19). These results suggest that the PKI domain is functionally important for the ABI protein phosphatases.
The PPI sequence is not only conserved in the PKS proteins but is also found in the cell cycle checkpoint kinase Chk1 from various organisms. In response to DNA damage or replication block, two upstream protein kinases, ATM and ATR, phosphorylate and activate Chk1, which in turn phosphorylates and inhibits the activities or enhances the degradation of the Cdc25 family of protein phosphatases required for cell cycle progression (34). The presence of a putative PPI sequence motif in Chk1 suggests that it may interact with Cdc25 or an unknown protein phosphatase.
ABI2 and ABI1 are well-known regulators of ABA signaling (35, 36), which is important for plant tolerance to several abiotic stresses including salt, drought, and freezing (37). ABA may exert its effect on salt tolerance through the interaction between ABI2 and SOS2. In this scenario, ABI2 would be a point of cross talk between the ABA pathway and the SOS pathway for ion homeostasis. Alternatively, ABI2 may have a role in the SOS pathway that is independent of ABA. In this model, SOS2 can be considered as a scaffold protein that holds SOS3 and ABI2 in one complex, which functions specifically in salt tolerance by responding to a distinct calcium signal triggered by sodium stress (Fig. 8). Similarly, PKS3 would serve as a scaffold for SCaBP5 and ABI2, and this complex would function specifically in ABA signaling (9) by responding to a specific calcium signal elicited by ABA. Consistent with this hypothesis, sos3 and sos2 mutants are specifically defective in salt tolerance but not in ABA responses (1, 38), whereas scabp5 and pks3 mutants are altered only in ABA sensitivity but not significantly in salt tolerance (9).
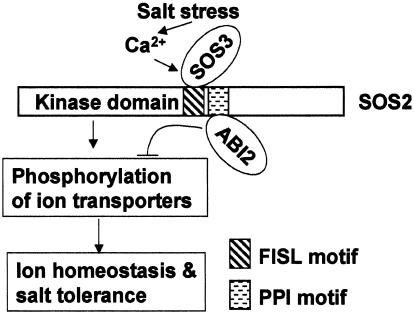
Fig. 8.
Model showing the involvement of SOS2 and its binding proteins SOS3 and ABI2 in salt tolerance. Salt stress elicits a calcium signal that is perceived by SOS3. SOS3 activates SOS2, which in turn phosphorylates ion transporters important for ion homeostasis and salt tolerance. ABI2 may deactivate SOS2 or dephosphorylate the target ion transporters of SOS2. ABI2 and SOS3 are shown to bind to SOS2 via the FISL and PPI motifs, respectively.
The functional relationship between SOS2 and ABI2 needs further investigation. Theoretically, SOS2 and ABI2 may control the phosphorylation status of each other, or they may regulate the phosphorylation status of common protein substrates. SOS2 did not phosphorylate ABI2 in vitro, nor did ABI2 dephosphorylate autophosphorylated SOS2 (data not shown). However, evidence suggests that SOS2 may be phosphorylated by an unknown upstream kinase (39), so it is still possible that ABI2 might dephosphorylate SOS2 that is phosphorylated by the upstream kinase. The protein phosphatase KAPP from Arabidopsis binds to the catalytic domain of receptor-like protein kinases (40, 41). It has been suggested that KAPP may dephosphorylate the receptor-like kinases (42).
It is also possible that ABI2 dephosphorylates proteins that are phosphorylated by SOS2 (Fig. 8). For example, SOS2 activates SOS1 by phosphorylation (8), and this may be countered by ABI2-catalyzed dephosphorylation. Consistent with a role of ABI2 in salt tolerance, we found that abi2 mutant seedlings are more tolerant to salt shock. The abi1 mutant plants are also more tolerant to salt shock, possibly because the abi1 mutation is dominant and thus masks the effect of the WT ABI2. The opposite activities of SOS2 and ABI2 are expected to be under tight regulation so that they do not occur simultaneously. Because SOS3 and ABI2 bind to adjacent sequences in SOS2, SOS3 and ABI2 are also in proximity. The close proximity of SOS3 to both SOS2 and ABI2 suggests that this calcium sensor may simultaneously regulate the opposing enzymes.
Acknowledgments
We thank Ms. Becky Stevenson for excellent technical assistance and Dr. André Jagendorf for critical reading of the manuscript. This work was supported by National Institutes of Health Grant R01 GM59138 and United States–Israel Binational Agricultural Research and Development Fund Grant IS-2971-98 (to J.-K.Z.).
Abbreviations: SOS2, salt overly sensitive 2; ABA, abscisic acid; ABI, ABA-insensitive; PPI, protein phosphatase interaction; PKS, protein kinase S; PKI, protein kinase interaction; SC, synthetic complete; β-gal, β-galactosidase; MS, Murashige and Skoog.
References
- 1.Zhu, J. K., Liu, J. & Xiong, L. (1998) Plant Cell 10, 1181–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu, J., Ishitani, M., Halfter, U., Kim, C. S. & Zhu, J. K. (2000) Proc. Natl. Acad. Sci. USA 97, 3730–3734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guo, Y., Halfter, U., Ishitani, M. & Zhu, J. K. (2001) Plant Cell 13, 1383–1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Knight, H., Trewavas, A. J. & Knight, M. R. (1997) Plant J. 12, 1067–1078. [DOI] [PubMed] [Google Scholar]
- 5.Halfter, U., Ishitani, M. & Zhu, J. K. (2000) Proc. Natl. Acad. Sci. USA 97, 3735–3740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shi, H., Ishitani, M., Kim, C. & Zhu, J. K. (2000) Proc. Natl. Acad. Sci. USA 97, 6896–6901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Qiu, Q. S., Guo, Y., Dietrich, M. A., Schumaker, K. S. & Zhu, J. K. (2002) Proc. Natl. Acad. Sci. USA 99, 8436–8441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Quintero, F. J., Ohta, M., Shi, H., Zhu, J. K. & Pardo, J. M. (2002) Proc. Natl. Acad. Sci. USA 99, 9061–9066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guo, Y., Xiong, L., Song, C. P., Gong, D., Halfter, U. & Zhu, J. K. (2002) Dev. Cell 3, 233–244. [DOI] [PubMed] [Google Scholar]
- 10.Moore, F., Weekes, J. & Hardie, D. G. (1991) Eur. J. Biochem. 199, 691–697. [DOI] [PubMed] [Google Scholar]
- 11.Maeda, T., Wurgler-Murphy, S. M. & Saito, H. (1994) Nature 369, 242–245. [DOI] [PubMed] [Google Scholar]
- 12.Sheen, J. (1996) Science 274, 1900–1902. [DOI] [PubMed] [Google Scholar]
- 13.Gaits, F., Shiozaki, K. & Russell, P. (1997) J. Biol. Chem. 272, 17873–17879. [DOI] [PubMed] [Google Scholar]
- 14.Koornneef, M., Reuling, G. & Karssen, C. M. (1984) Physiol. Plant 61, 377–383. [Google Scholar]
- 15.Leung, J., Bouvier-Durand, M., Morris, P. C., Guerrier, D., Chefdor, F. & Giraudat, J. (1994) Science 264, 1448–1452. [DOI] [PubMed] [Google Scholar]
- 16.Meyer, K., Leube, M. P. & Grill, E. (1994) Science 264, 1452–1455. [DOI] [PubMed] [Google Scholar]
- 17.Leung, J., Merlot, S. & Giraudat, J. (1997) Plant Cell 9, 759–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rodriguez, P. L., Benning, G. & Grill, E. (1998) FEBS Lett. 421, 185–190. [DOI] [PubMed] [Google Scholar]
- 19.Sheen, J. (1998) Proc. Natl. Acad. Sci. USA 95, 975–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gosti, F., Bertauche, N., Vartanian, N. & Giraudat, J. (1995) Mol. Gen. Genet. 246, 10–18. [DOI] [PubMed] [Google Scholar]
- 21.Merlot, S., Gosti, F., Guerrier, D., Vavasseur, A. & Giraudat, J. (2001) Plant J. 25, 295–303. [DOI] [PubMed] [Google Scholar]
- 22.Soderman, E., Mattsson, J. & Engstrom, P. (1996) Plant J. 10, 375–381. [DOI] [PubMed] [Google Scholar]
- 23.de Bruxelles, G. L., Peacock, W. J., Dennis, E. S. & Dolferus, R. (1996) Plant Physiol. 111, 381–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pei, Z. M., Kuchitsu, K., Ward, J. M., Schwarz, M. & Schroeder, J. I. (1997) Plant Cell 9, 409–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Strizhov, N., Abraham, E., Okresz, L., Blickling, S., Zilberstein, A., Schell, J., Koncz, C. & Szabados, L. (1997) Plant J. 12, 557–569. [DOI] [PubMed] [Google Scholar]
- 26.Murata, Y., Pei, Z. M., Mori, I. C. & Schroeder, J. (2001) Plant Cell 13, 2513–2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim, J., Harter, K. & Theologis, A. (1997) Proc. Natl. Acad. Sci. USA 94, 11786–11791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bai, C. & Elledge, S. J. (1996) Methods Enzymol. 273, 331–347. [DOI] [PubMed] [Google Scholar]
- 29.Gong, D., Gong, Z., Guo, Y., Chen, X. & Zhu, J. K. (2002) J. Biol. Chem. 277, 28340–28350. [DOI] [PubMed] [Google Scholar]
- 30.Gong, D., Zhang, C., Chen, X., Gong, Z. & Zhu, J. K. (2002) J. Biol. Chem. 277, 42088–42096. [DOI] [PubMed] [Google Scholar]
- 31.Cohen, G. B., Ren, R. & Baltimore, D. (1995) Cell 80, 237–248. [DOI] [PubMed] [Google Scholar]
- 32.Kay, B. K., Williamson, M. P. & Sudol, M. (2000) FASEB J. 14, 231–241. [PubMed] [Google Scholar]
- 33.Kerk, D., Bulgrien, J., Smith, D. W., Barsam, B., Veretnik, S. & Gribskov, M. (2002) Plant Physiol. 129, 908–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sagata, N. (2002) Science 298, 1905–1907. [DOI] [PubMed] [Google Scholar]
- 35.Giraudat, J. (1995) Curr. Opin. Cell Biol. 7, 232–238. [DOI] [PubMed] [Google Scholar]
- 36.Leung, J. & Giraudat, J. (1998) Annu. Rev. Plant Physiol. Plant Mol. Biol. 49, 199–222. [DOI] [PubMed] [Google Scholar]
- 37.Zhu, J. K. (2002) Annu. Rev. Plant Biol. 53, 247–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu, J. & Zhu, J. K. (1998) Science 280, 1943–1945. [DOI] [PubMed] [Google Scholar]
- 39.Gong, D., Guo, Y., Jagendorf, A. T. & Zhu, J. K. (2002) Plant Physiol. 130, 256–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stone, J. M., Collinge, M. A., Smith, R. D., Horn, M. A. & Walker, J. C. (1994) Science 266, 793–795. [DOI] [PubMed] [Google Scholar]
- 41.Li, J., Smith, G. P. & Walker, J. C. (1999) Proc. Natl. Acad. Sci. USA 96, 7821–7826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shah, K., Russinova, E., Gadella, T. W., Jr., Willemse, J. & De Vries, S. C. (2002) Genes Dev. 16, 1707–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]