Abstract
Stomatal guard cells play a key role in the ability of plants to survive on dry land, because their movements regulate the exchange of gases and water vapor between the external environment and the interior of the plant. The walls of these cells are exceptionally strong and must undergo large and reversible deformation during stomatal opening and closing. The molecular basis of the unique strength and flexibility of guard cell walls is unknown. We show that degradation of cell wall arabinan prevents either stomatal opening or closing. This locking of guard cell wall movements can be reversed if homogalacturonan is subsequently removed from the wall. We suggest that arabinans maintain flexibility in the cell wall by preventing homogalacturonan polymers from forming tight associations.
Life on dry land requires that organisms have tight control over the exchange of water and gases between themselves and the environment. In plants this control is achieved by virtue of a waterproof cuticular covering perforated with pores of adjustable aperture (stomata). Opening stomatal pores is necessary for the exchange of oxygen and carbon dioxide during photosynthesis, but it also allows water vapor to escape. A large deciduous tree, for example, may transpire as much as 400 liters of water per day through its stomata (1). Under conditions of limiting water, stomata are closed to prevent the dehydration of cells within the plant. Reversible changes in the size and shape of the pairs of guard cells that form the stomatal pore regulate its aperture.
Guard cell expansion and contraction are driven by changes in internal hydrostatic (or turgor) pressure. Turgor pressures within guard cells are extremely high, reaching values on the order of 5 MPa, equivalent to 50 times atmospheric pressure (2), which is ≈10 times greater than the pressure found in most other plant cells. During stomatal opening, guard cell pressure rises, causing the cells to inflate by up to 70% in volume and to bend apart. During closing, guard cell turgor drops, and the cells shrink to their original size. Guard cell walls must combine great physical strength with remarkable elasticity to fulfill the demands of their role.
The molecular basis of the physical properties of plant cell walls, in general, and the physical properties of guard cells, in particular, remain poorly understood (3). Plant cell walls are mostly composed of polysaccharides with a small amount of structural protein. The structural framework of the wall is built around strong crystalline cellulose microfibrils that are bound to one another by a coating of hemicellulose polymers to form a cohesive network (4). Pectins are a complex group of acidic polysaccharides that form a network coextensive with that of cellulose and hemicelluloses. Pectins may account for up to 30% of the dry weight of a plant cell wall, and guard cells are particularly rich in these polymers (5). Pectins are composed of a mixture of linear and branched polymers characterized by the presence of acidic sugar residues (galacturonic acid) in their backbone, which allows them to form complexes by electrostatic interactions through calcium ions (6, 7). Linear chains of (1–4)-α-d-galacturonic acid (homogalacturonan) form a major component of pectins, and these can associate to form rigid structures. The carboxyl groups of galacturonosyl residues in homogalacturonan are often substituted with an esterified methyl group, and the degree of methyl esterification of the polymer influences its ability to form tight gels (8). Other pectic polymers are more highly branched. For example, rhamnogalacturonan 1 (RG-1) is extensively decorated with galactan and arabinan side chains, which in turn are often substituted with terminal phenolic esters, particularly feruloyl or coumaroyl esters, which can dimerize oxidatively to form links between polymers. The roles of these different pectic polymers in cell walls remain unclear. Here we present data that arabinan chains play a key role in determining guard cell wall flexibility, and we suggest that they do this by maintaining fluidity within the pectin network in the walls.
Materials and Methods
Preparation of Stomata. Epidermal strips were peeled from the abaxial surface of mature leaves of 6-week-old Commelina communis and placed in 10 mM KCl/0.1 mM CaCl2. Strips were then trimmed and cut to size, ≈5 mm2. For stomatal opening experiments, strips were incubated in the dark for 1 h in 1 ml of 10 mM KCl/0.1 mM CaCl2 containing enzymes. Strips were then placed in 75 mM KCl/1 μM fusicoccin and left to open for 2 h. For closing, strips were initially allowed to open in 75 mM KCl in the light for 2 h, then treated with enzyme for a further 1 h, and finally closed with 10 mM KCl/0.1 mM CaCl2/1 μM abscisic acid (ABA) for 10 min or 0.5 M mannitol for 5 min in the dark.
Enzyme Treatments. Highly purified enzymes with well defined properties were purchased from Megazyme (Bray, Ireland). These enzymes included endo-1,4-β-galactanase, endoarabinanase, and endopolygalacturonanase, all reported to be purified to single bands as seen by SDS gel electrophoresis. Pectinesterase P5400 was purchased from Sigma-Aldrich. Purified recombinant feruloyl esterase FaeA (9) was a kind gift from C. Faulds (Institute of Food Research, Norwich, U.K.). Enzyme incubations were carried out in 10 mM KCl/0.1 mM CaCl2 before inducing stomatal opening, or in 75 mM KCl for closing experiments, for 1 h at 20°C. All enzymes were used at 10 units/ml, except for feruloyl esterase, which was used at 1 unit/ml. Reducing sugars released by enzyme treatments were quantified by using 2-cyanoacetamide as described (10). The release of ferulic compounds from epidermal strips by feruloyl esterase was monitored by a spectrophotometric method as described (9).
Epitope Protection Assays. LM5 and LM6 monoclonal antibodies were a kind gift from P. Knox (University of Leeds, Leeds, U.K.). Pieces (2 mm2) of epidermal strip were incubated in 2 ml of 1% Marvel milk powder (Premier Brands, Spalding, U.K.) in PBS containing a 1:20 dilution of either LM5 or LM6 monoclonal antibodies overnight in the dark. Strips were washed three times in PBS and then incubated in 1 ml of 10 mM KCl/0.1 mM CaCl2 containing enzymes at 10 units/ml. After enzyme treatment, strips were transferred into 1 ml of 75 mM KCl/1 μM fusicoccin for 2 h before measuring stomatal apertures.
Immunofluorescence Studies. Epidermal strips were fixed overnight in 4% paraformaldehyde, dehydrated in a graded ethanol series, and then infiltrated with LR White resin (London Resin, Reading, U.K.) for several days. Finally, the strips were embedded in LR White and polymerized overnight at 50°C. Sections (0.5 μm) were cut by using a Leica Ultracut UCT (Leica, Deerfield, IL) and transferred to eight-well slides (ICN). Sections were blocked in 1% Marvel milk powder in PBS for at least 30 min and then incubated with LM6, 1:20 dilution; JIM5, 1:20 dilution; or JIM7, 1:50 dilution, overnight at 4°C. After three washes in PBS/1% Marvel milk powder, strips were incubated with a 1:150 dilution of FITC-conjugated anti-rat secondary antibody (F6258, Sigma-Aldrich). Sections were washed three times in PBS/1% Marvel milk powder before being mounted in CitiFluor (Agar Scientific, Stansted, U.K.) and examined by fluorescence microscopy.
Image Capture and Analysis. Epidermal strips were examined by bright-field microscopy with an Optiphot light microscope (Nikon 104). Stomatal apertures were measured by using lucia g version 3.52a software (Laboratory Imaging, Prague). Fluorescent images were captured by using a CoolSnap digital camera (RS Photometrics, Trenton, NJ) attached to a Microphot FXA Nikon microscope and analyzed with coolsnap 1.2 software (Roper Scientific, Trenton, NJ).
Cell Wall Monosaccharide Analysis. Epidermal strips were taken from the abaxial side of leaves of 6-week-old C. communis plants and stirred slowly in liquid phenol (P-4682, Sigma) for 20 min. Strips were washed four times in 95% ethanol to remove the phenol and then left to dry. Duplicate 1-mg samples were hydrolyzed with 2 M trifluoroacetic acid for 4 h at 100°C and separated by high-performance anion-exchange chromatography on a Dionex Carbopac PA-10 column with integrated amperometry detection. The separated monosaccharides were quantified by using external calibration with an equimolar mixture of nine monosaccharide standards (arabinose, fucose, galactose, galacturonic acid, glucose, glucuronic acid, mannose, rhamnose, and xylose) that were subjected to acid hydrolysis in parallel with the samples.
Results
In our experiments we investigated the effects of modifying specific cell wall polymers on the ability of stomata to open or close. We used epidermal strips from C. communis, a species with large, abundant guard cells that has served as a model for stomatal studies. Guard cells remain functional in isolated epidermal strips and will respond to signals such as hormones and light in a manner similar to that seen in intact leaves (11). We made use of two compounds that affect stomatal aperture. The first is fusicoccin, a fungal toxin that increases the rate of proton pumping at the plasma membrane (12) leading to stomatal opening. Although fusicoccin is not a normal physiological signal in plants, it is an effective and reliable tool for inducing stomatal opening. The second is ABA, a plant hormone involved in drought stress responses in plants, which induces stomatal closing. We also used external osmotica (0.5 M mannitol) to physically remove internal cell turgor without reliance on cell physiology. Modification of the cell wall was achieved by enzymatic digestion with well defined, highly purified enzymes, and enzyme activity was assessed by monitoring the release of reducing sugars after treatments. Cell viability after enzyme digestion was assessed by fluorescein diacetate staining (13). We found that several enzymes that digested cellulose or hemicelluloses had no detectable effect on guard cell function in our assays. For example, incubation with cellulase, which hydrolyzes (1–4)-β-glucans, led to a generalized disruption of nonguard cells in the epidermal strips, but the stomata opened normally in response to fusicoccin treatment (data not shown). Similarly, xylanase (which will degrade arabinoxylans, the major hemicellulose in commelinoid species) had a general disruptive effect on guard cells, eventually causing lysis, but the stomata functioned normally until the point of cell death (data not shown). In contrast, many pectin-degrading enzymes had profound effects on stomatal function.
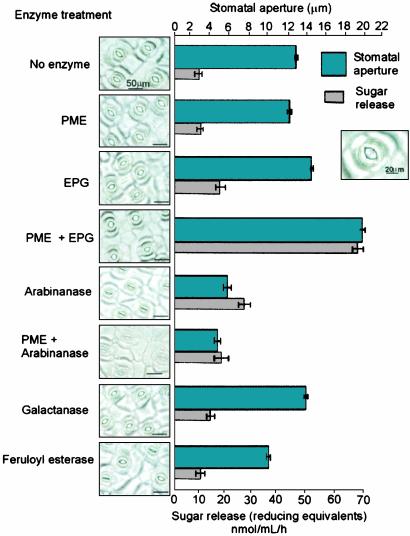
Hydrolysis of Cell Wall Homogalacturonan Increases Stomatal Aperture. Fusicoccin-induced stomatal opening was not affected by digestion with either endopolygalacturonase (EPG) or pectin methylesterase (PME) (Fig. 1). However, we found that digestion of the cell walls with a combination of these two enzymes caused the stomata to open far wider than normal because of increased bending of the guard cell walls. EPG action is known to be highly active in degrading unesterified homogalacturonan but much less effective on the methyl-esterified form of this polymer (14). Analysis of the release of sugar from the epidermal strips during incubation with these enzymes indicated that, although PME alone released little or no sugar, it increased the sugar release catalyzed by EPG >3-fold in a combined incubation, suggesting that much of the homogalacturonan in these walls exists in a methyl-esterified form. It has been reported that oligogalacturonides (fragments of homogalacturonan) can reduce stomatal aperture in C. communis by acting as signal molecules (15). It is unlikely that these oligosaccharides play this role in our results because, in this case, homogalacturonan degradation causes the stomata to open wider, and we suggest this is through a direct effect on wall structure.
Fig. 1.
Enzymes that modify pectins alter guard cell function. Epidermal strips were incubated with 10 units of specific pectin-modifying enzymes for 1 h before treatment with fusicoccin to stimulate stomatal opening. Representative images from treated epidermal strips are presented, along with values for final stomatal apertures after fusicoccin treatment; data are averages and standard errors from measurements of a total of 100 guard cell pairs (from three separate strips) for each treatment. Data are also presented for the amount of sugar (as reducing sugar equivalents) released by each incubation; data are averages and standard errors for three measurements. The distance measured after fusicoccin treatment to obtain final stomatal aperture is shown in Inset. Overall experiments were repeated five times with similar results.
Hydrolysis of Cell Wall Arabinan Prevents Stomatal Opening. The most dramatic effect was observed when epidermal strips were incubated with (1–5)-α-l-arabinanase (arabinanase) after which the stomatal pore failed to open (Fig. 1). Arabinanase is an enzyme specific in its action to the endohydrolytic cleavage of (1–5)-α-l-arabinans that typically occur as side chains in RG-1 but is not active on terminal arabinosyl residues commonly found in hemicelluloses (16). When epidermal strips were incubated with arabinanase and PME in combination, the reduction in fusicoccin-induced opening was even greater than for strips treated with only arabinanase, suggesting a synergistic effect of these two enzymes. In contrast with arabinanase, incubation with (1–4)-β-d-galactanase (galactanase) had no discernible effect on guard cell opening. We found that treatment with feruloyl esterase (an enzyme that removes feruloyl groups) also inhibited fusicoccin-induced stomatal opening but to a lesser degree than that observed with arabinanase treatment. Data indicating sugar release after digestion with these enzymes is also shown in Fig. 1. Fusicoccin-induced stomatal opening was also found to be inhibited by arabinanase digestion in epidermal strips from Arabidopsis thaliana (data not shown), indicating that these observations are not exclusive to commelinoid species.
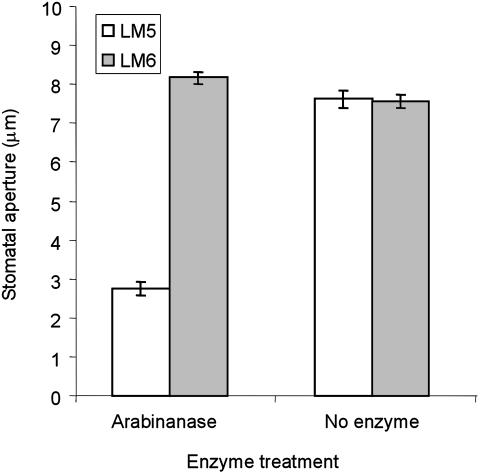
Arabinanase-Induced Wall “Locking” Is Specifically Blocked by Arabinan-Binding Antibodies. To determine whether the observed arabinanase-induced locking of the wall was due to arabinan hydrolysis, we undertook immunoprotection assays making use of two monoclonal antibodies, LM5 and LM6. These antibodies were raised against short-chain linear oligomers of (1–4)-β-d-galactan (17) and (1–5)-α-l-arabinan (18), respectively, and have been shown to bind specifically to linear stretches of three or more linked residues but do not recognize single galactosyl or arabinosyl residues. Such linear stretches of galactan and arabinan are generally associated with RG-I in the cell wall (4). These antibodies were used in epitope-protection assays to assess whether antibody binding could prevent the locking of the cell wall by arabinanase. In these experiments, epidermal strips were first treated with either LM5 or LM6 monoclonal antibodies before being digested with enzymes and then treated with fusicoccin to induce stomatal opening. When epidermal strips that had been incubated with the galactan-binding LM5 antibody were digested with arabinanase, they again failed to open in response to fusicoccin, indicating that this antibody was unable to prevent the arabinanase effects (Fig. 2). In contrast, incubation of epidermal strips with arabinan-binding antibody LM6 prevented arabinanase treatment from causing the wall to lock, and the stomata were able to open normally in response to fusicoccin. We found that binding of the antibodies to epidermal strips had no direct effect on stomata, which opened normally in response to fusicoccin. This specific blocking of the effects of arabinanase treatment by an arabinan-binding antibody strongly supports the case that arabinans are the targets of the arabinanase effects on guard cell walls.
Fig. 2.
Monoclonal antibodies recognizing arabinans prevent arabinanaseinduced locking of guard cell walls. Epidermal strips were incubated with monoclonal antibodies recognizing galactan (LM5), or arabinan side chains (LM6), before digestion with enzymes and subsequent fusicoccin treatment. Final stomatal apertures after various treatments are presented; data are averages and standard errors from measurements of 100 guard cell pairs (from three separate strips) for each treatment. Overall experiments were repeated three times with similar results.
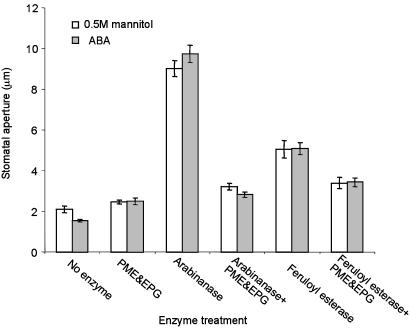
Arabinanase-Induced Locking of Guard Cell Walls Depends on the Presence of Homogalacturonan. To eliminate the possibility that arabinanase was indirectly rendering guard cells insensitive to opening stimuli, we undertook a different approach. Epidermal strips were first treated with 75 mM KCl in the light to induce stomatal opening. Once stomata were fully open (≈11-μm aperture), the strips were treated with enzymes before inducing closure with ABA or 0.5 M mannitol. The closing observed with 0.5 M mannitol is a purely physical event and a result of this osmoticum removing turgor pressure from the cells. This mechanism is hence independent of signal transduction processes. Fig. 3 shows that untreated stomata that had been opened by high concentrations of potassium and light successfully closed in response to ABA or 0.5 M mannitol. Stomata treated with a combination of PME and EPG also closed normally in response to either ABA or 0.5 M mannitol. Open stomata treated with arabinanase or feruloyl esterase, however, failed to close in response to either ABA or 0.5 M mannitol. Again, this effect was much more pronounced with arabinanase than with feruloyl esterase treatment. When stomata that had been allowed to open and then were treated with arabinanase or feruloyl esterase were subsequently treated with a combination of PME and EPG (Fig. 3, arabinanase/PME plus EPG and feruloyl esterase/PME plus EPG, respectively), they closed in response to both ABA and 0.5 M mannitol. We similarly found that the “locking” effects of arabinanase treatment could be reversed by subsequent treatment with 10 mM diaminocyclohexane tetraacetic acid, allowing the stomata to close in response to osmotica (data not shown). Diaminocyclohexane tetraacetic acid is a strong chelator of metal cations and is known to disrupt calcium-bonded interactions between homogalacturonan polymers in cell walls (4). Therefore, apparently the disruption or removal of homogalacturonan in or from the cell wall is sufficient to overcome the locking effects observed with arabinanase, or feruloyl esterase treatment.
Fig. 3.
Arabinanase and feruloyl esterase prevent stomatal closure, and this is reversed by HGA disruption. Epidermal strips were incubated with 75 mM KCl in the light to induce stomatal opening and then incubated with 10 units of each enzyme before being treated with ABA or 0.5 M mannitol to induce closing. Final apertures of stomata in enzyme-treated epidermal strips after treatment with ABA or 0.5 M mannitol are presented. Data are averages and standard errors from a total of 100 measurements taken from three separate strips. Overall experiments were repeated five times with similar results.
Commelina Guard Cell Walls Are Rich in Homogalacturonan and Contain Arabinans. Analysis of epidermal strip cell wall monosaccharide composition, shown in Fig. 4A, revealed that galacturonic acid represents >30% of the sugars in these walls. These levels of galacturonic acid are comparable to those generally reported in dicot cell walls and indicate that the walls of epidermal cells in Commelina are rich in pectin.
Fig. 4.
Guard cell walls are rich in pectin and contain arabinan epitopes. (A) Cell walls from epidermal strips were hydrolyzed in trifluoroacetic acid, and the monosaccharides released were analyzed by anion-exchange chromatography. (B–F) Resin-embedded sections (0.5 μm thick) of epidermal strips were probed with monoclonal antibodies that bind arabinans (LM6), esterified HGA (JIM7), or unesterified HGA (JIM5). Labeling was detected with a FITC-labeled secondary antibody and visualized by fluorescence microscopy. The images shown represent LM6 (1:20) (B), control probed with secondary antibody only (C), an unlabeled section viewed under UV light to reveal autofluorescent material corresponding to endogenous wall phenolic compounds (D), JIM7 (1:50) (E), and JIM5 (1:20) (F). E, epidermal cells; S, subsidiary cells; G, guard cells. (Bars = 10 μm.)
Immunolocalization studies were undertaken to examine the distribution of arabinan and homogalacturonan (HGA) epitopes in guard cell walls. Immunofluorescence studies using FITC-conjugated anti-rat secondary antibodies revealed that LM6 (Fig. 4B) produced low levels of fluorescence along the inner, plasma-membrane face of the cell walls throughout the epidermal strip, indicating the presence of arabinan chains in these walls. In the absence of LM6 or an alternative primary antibody, a small degree of autofluorescence could be detected, predominantly in the walls surrounding the guard cells (Fig. 4C). This signal, however, could be clearly differentiated from the signal observed with the FITC-conjugated antibody. Fig. 4D represents a direct comparison of the same strip used in Fig. 4B visualized with UV light. Bright blue autofluorescence is readily evident throughout the cell walls in an epidermal strip, indicating the presence of high levels of ferulic acid esters that have a characteristic blue fluorescence (19). Fig. 4E shows abundant labeling throughout the cell walls in the epidermal strips labeled with JIM7, a monoclonal antibody that recognizes methyl-esterified HGA (20). Similar studies were undertaken with JIM5, an antibody that preferentially binds to unesterified pectins (Fig. 4F). Labeling in guard cells predominates in the cuticular ledges and the outer cell wall. Indeed, labeling with JIM5 was consistently brighter throughout the outer walls of the subsidiary and epidermal cells in comparison with the ventral, dorsal, and inner walls. The JIM5 epitope was also found predominantly at cell corners where neighboring cells convene. The data presented in this section clearly demonstrate that epidermal cell walls from C. communis are rich in pectins and that the walls of guard cells and subsidiary cells contain arabinan epitopes and feruloyl esters.
Discussion
The experiments presented here directly assess the effects of a range of specific cell wall-degrading enzymes on guard cell function. In a recent study, stomata in leaves of transgenic apple trees overexpressing EPG showed impaired responses to darkness and ABA (21). However, these effects were the result of severe malformation of epidermal and guard cells rather than specific alterations in guard cell wall composition. In our studies, the fact that two different wall-active enzymes (arabinanase and feruloyl esterase) impair guard cell function and that these effects can be reversed by the action of other wall-active enzymes (PME and EPG) strongly suggests that the observed effects are the direct result of wall modifications rather than interference with other aspects of cell physiology. The specificity of the effects described in this study are compelling not only because arabinanase (an enzyme that cleaves linear arabinans) treatment prevented either stomatal opening or closing, but also because this effect was blocked by preincubating the cells with a monoclonal antibody known to bind specifically to linear arabinan chains.
Our data suggest that pectic arabinan is the most likely target for the observed effects of arabinanase treatment described in this article. Plant cell walls contain a number of polymers with arabinosyl side chains, including glucuronoarabinoxylan (GAX), which is generally considered to be an abundant hemicellulose polymer in commelinoid plants. As such, it seems possible that the arabinanase effects that we report here might be the result of the action of this enzyme on GAX. Several observations argue against this possibility. First, arabinosyl groups on GAX are typically present as single 1,3-linked α-arabinosyl side chains, and these groups are not targets for either the arabinanase or the LM6 monoclonal antibody used in this study. Both the enzyme and the antibody recognize 1-5-α-arabinan chains, which are predominantly found as side chains of RG-1. Second, we have found that arabinanase treatment has a similar effect, preventing stomatal opening in epidermal strips from A. thaliana, a species containing very low levels of GAX (22) and high levels of pectin.
In general, commelinoid plants such as C. communis are considered to be pectin-poor when compared with dicot plants (4). However, published data for Tradescantia fluminensis (a close relative of C. communis) show that galacturonic acid accounts for almost 20% of total cell wall sugars (23), indicating that pectins must make up at least this amount of the cell wall. This percentage compares well with dicots such as potato, in which, in the same report, galacturonic acid makes up 21.5% of wall monosaccharides. In the present work we show that galacturonic acid makes up >30% of wall sugars in Commelina epidermal strips, indicating that these cell walls are enriched with pectins. These values are considerably higher than those published for Tradescantia, but it is worth noting that the Tradescantia data are derived from stem internodes, whereas ours are from leaf epidermis. Given the differences in function in these tissues, such discrepancies in composition may not be surprising.
Our data suggest that arabinans maintain flexibility in guard cell walls, and preliminary data in Arabidopsis suggest that these observations are not unique to commelinoid species. Based on our results, we propose a model (Fig. 5) in which arabinan side chains occur in RG-1 domains associated with HGA. The presence of these arabinan chains provides steric hindrance such that HGA domains are unable to attain the spatial proximity required to form the rigid associations characteristic of these polymers. Removal of the arabinans in our experiments allows HGA polymers to coalesce and form tightly associated structures, probably through calcium-linked chain packing, making the wall more rigid and rendering the guard cells incapable of deforming in response to changes in cell turgor. This hypothesis is further strengthened by the observation that incubation with a combination of PME (an enzyme that removes methyl esters from carboxyl groups of HGA) and arabinanase is even more effective at preventing stomatal opening than arabinanase treatment alone. In our model, more highly charged HGA domains in the pectin can form a tighter gel once freed from the hindrance of the arabinan chains. We suggest that feruloyl esters may have a role in guard cell wall flexibility by providing crosslinks between arabinans and other wall polymers. Previous attempts to assign specific functional roles to pectic polymers in plant cell walls have proved difficult. This study, however, demonstrates a unique role for arabinans in determining the physical and functional properties of guard cell walls.
Fig. 5.
A model of the role of arabinans in maintaining pectin fluidity. Arabinan side chains of RG-1 provide steric hindrance to the association of neighboring domains of HGA. Digestion with arabinanase removes the side chains, allowing HGA domains to associate through calcium crosslinked chain packing.
Acknowledgments
We thank J. P. Knox for providing LM5 and LM6 monoclonal antibodies and for discussions of the data, C. B. Faulds for providing the feruloyl esterase used in this study, and E. A. C. MacRobbie, D. J. Bowles, and D. A. Rees for invaluable advice on the manuscript.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: EPG, endopolygalacturonanase; PME, pectin methyl esterase; RG-1, rhamnogalacturonan 1; ABA, abscisic acid; HGA, homogalacturonan.
References
- 1.Raven, P. H., Evert, R. H. & Eichhorn, S. E. (1999) Biology of Plants (Freeman, New York), 6th Ed.
- 2.Franks, P. J., Buckley, T. N., Shope, J. C. & Mott, K. A. (2001) Plant Physiol. 125, 1577–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roberts, K. (2001) Plant Physiol. 125, 127–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carpita, N. C. & Gibeaut, D. M. (1993) Plant J. 3, 1–30. [DOI] [PubMed] [Google Scholar]
- 5.Raschke, K. (1979) Encyclopedia of Plant Physiology, eds. Hampt, W. & Feinlieb, M. E. (Springer, Berlin), Vol. 7.
- 6.Willats, W. G. T., McCartney, L., Mackie, W. & Knox, J. P. (2001) Plant Mol. Biol. 47, 9–27. [PubMed] [Google Scholar]
- 7.Ridley, B. L., O'Neill, M. A. & Mohnen, D. A. (2001) Phytochemistry 57, 929–967. [DOI] [PubMed] [Google Scholar]
- 8.Powell, D. A., Morris, E. R., Gidley, M. J. & Rees, D. A. (1982) J. Mol. Biol. 155, 517–531. [DOI] [PubMed] [Google Scholar]
- 9.Ralet, M. C., Faulds, C. B., Williamson, G. & Thibault, G. F. (1994) Carbohydr. Res. 263, 257–269. [DOI] [PubMed] [Google Scholar]
- 10.Gross, K. C. (1982) Hortscience 17, 933–934. [Google Scholar]
- 11.MacRobbie, E. A. C. (1981) J. Exp. Bot. 32, 563–572. [Google Scholar]
- 12.Baunsgaard, L., Fuglsang, A. T., Jahn, T., Korthout, H. A., de Boer, A. H. & Palmgren, M. G. (1998) Plant J. 13, 661–667. [DOI] [PubMed] [Google Scholar]
- 13.Larkin, P. J. (1976) Planta 128, 213–216. [DOI] [PubMed] [Google Scholar]
- 14.Daas, P. J. H., Voragen, A. G. J. & Schols, H. A. (2001) Biopolymers 58, 195–203. [DOI] [PubMed] [Google Scholar]
- 15.Lee, S., Choi, H., Suh, S., Doo, I. S., Oh, K. Y., Choi, E. J., Schroeder Taylor, A. T., Low, P. S. & Lee, Y. (1999) Plant Physiol. 121, 147–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lerouge, P., O'Neill, M. A., Darvill, A. G. & Albersheim, P. (1993) Carbohydr. Res. 243, 373–378. [DOI] [PubMed] [Google Scholar]
- 17.Jones, L., Seymour, G. B. & Knox, J. P. (1997) Plant Physiol. 113, 1405–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Willats, W. G. T., Marcus, S. E. & Knox, J. P. (1998) Carbohydr. Res. 308, 149–152. [DOI] [PubMed] [Google Scholar]
- 19.Parker, M. L., Ng, A., Smith, A. C. & Waldron, K. W. J. (2000) Agric. Food Chem. 48, 6284–6291. [DOI] [PubMed] [Google Scholar]
- 20.Knox, J. P., Linstead, J., King, J., Cooper, C. & Roberts, K. (1990) Planta 181, 512–521. [DOI] [PubMed] [Google Scholar]
- 21.Atkinson, R. G., Schroder, R., Hallett, I. C., Cohen, D. & MacRae, E. A. (2002) Plant Physiol. 129, 122–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zablackis, E., Huang, J., Muller, B., Darvill, A. G. & Albersheim, P. (1995) Plant Physiol. 107, 1129–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harris, P. J., Kelderman, M. R., Kendon, M. F. & Mckenzie, R. J. (1997) Biochem. Syst. Ecol. 25, 167–179. [Google Scholar]