Abstract
Lateral root base nodulation on the tropical, semiaquatic legume Sesbania rostrata results from two coordinated, Nod factor-dependent processes: formation of intercellular infection pockets and induction of cell division. Infection pocket formation is associated with cell death and production of hydrogen peroxide. Pharmacological experiments showed that ethylene and reactive oxygen species mediate Nod factor responses and are required for nodule initiation, whereby induction of division and infection could not be uncoupled. Application of purified Nod factors triggered cell division, and both Nod factors and ethylene induced cavities and cell death features in the root cortex. Thus, in S. rostrata, ethylene and reactive oxygen species act downstream from the Nod factors in pathways that lead to formation of infection pockets and initiation of nodule primordia.
In the Rhizobium-legume symbiosis, root nodules are formed that provide a niche for bacterial nitrogen fixation. Rhizobia can enter the plant via several mechanisms (1), of which the best documented is invasion of root hairs located behind the root tip. The rhizobia induce susceptible root hairs to curl, colonize the curl, and trigger formation of an intracellular infection thread that grows toward a nodule primordium, where bacteria are released in plant cells and differentiate into bacteroids (2). Both nodule primordium initiation and preparation of the invasion track are initiated by bacterial Nod factors (lipochitooligosaccharides; ref. 2). Perception of Nod factors, presumably via receptor complexes at the plant plasma membrane (3), leads to a cascade of physiological changes and affects gene expression (4).
Certain features of rhizobial invasion are reminiscent of pathogen infection (5); molecules involved in pathogen responses, such as ethylene (6) and reactive oxygen species (ROS; ref. 7), also intervene in nodulation. Ethylene plays mostly a negative role in legume symbiosis, probably by restricting infection. In Medicago sativa, nodulation is inhibited by exogenous ethylene and stimulated by an ethylene synthesis inhibitor (8), whereas an ethylene-insensitive mutant of Medicago truncatula is hyperinfected by Sinorhizobium meliloti (9). In Pisum sativum, exogenous ethylene inhibited nodulation (10), and the nodulation-defective mutant (R50, sym16) is rescued by inhibitors of ethylene (11). In Vicia faba, Nod factors trigger enhanced ethylene levels that restrict infection and also in Lotus japonicus, invasion is blocked by excessive ethylene (12). Ethylene negatively modulates Nod factor-triggered calcium signaling in root hairs of Medicago truncatula and the induction of two nodulin genes (13). Endogenous ethylene also contributes to positioning of the nodule primordia opposite of protoxylem poles (14).
An oxidative burst is one of the earliest plant responses to many pathogen elicitors (7). Data for a role of ROS in nodulation are sparse, but interesting. In Medicago sativa, superoxide radicals are present in infection threads, and H2O2 was located in walls of infected cells and of some infection threads (15). Indirect evidence suggests that H2O2 may be involved in crosslinking of glycoproteins in the matrix of infection threads of P. sativum (16). In addition, ROS production mediates expression of the rip1 nodulin gene in Medicago truncatula (17).
All these data deal with nodulation via root hair invasion. In a far less characterized process, bacteria invade intercellularly, in the fissure region at bases of lateral or adventitious roots. Intercellular entry and cortex colonization are the rule in a number of tropical flooding-tolerant legumes, where root hairs are sparse. The semiaquatic legume Sesbania rostrata belongs to that group. When inoculated with Azorhizobium caulinodans, hydroponic roots of S. rostrata develop nodules at lateral root bases. On the stem, dark-green nodules are formed at the bases of adventitious rootlets. During nodule initiation, cortical infection pockets are occupied by proliferating bacteria; this process depends on Nod factors (18). From infection pockets, inter- and intracellular infection threads guide the bacteria toward the nodule primordia (19, 20).
In the physiology of water-adapted plants, ethylene plays a special role, often as growth stimulator. In deepwater rice, for instance, submergence stimulates ethylene-mediated stem elongation and adventitious root outgrowth (21). Also in S. rostrata, ethylene controls features of growth and nodulation. Previously, we have shown that ethylene interferes in the nodule type, which is determinate or indeterminate, depending on position or environment (22). Here, we provide experimental evidence that ROS and ethylene are part of a Nod factor-induced signal cascade important for nodule primordium initiation and for intercellular bacterial colonization of the cortex at lateral root bases.
Materials and Methods
Bacteria and Nod Factors. Azorhizobium caulinodans ORS571 (23), ORS571-V44 (24), ORS571(pBBR5-hem-gfp5-S65T) (W.D. and M.H., unpublished results), ORS571(pRG960SD-32) (25), and ORS571(pRG290-12::T20) (26) were grown as described (27). Expression of nod genes was induced according to ref. 28. Mixtures of vaccenoylated and palmitoylated Nod factors (28) were added at final concentrations of 10–9 M or 5.10–8 M, and chitopentaose at a concentration of 10–8 to 10–9 M.
Plant Growth and Inoculations. S. rostrata Brem plants were grown and treated as described (22), except that, in the Norris medium, Cl– was replaced by 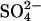 . Tubes were wrapped in aluminum foil 1 day before treatment with inhibitors.
. Tubes were wrapped in aluminum foil 1 day before treatment with inhibitors.
Staining and Microscopy. Root hairs were visualized according to D'Haeze et al. (27). β-Glucuronidase (GUS) assays and toluidine blue staining were as described (18) and Evans blue staining according to Mergemann and Sauter (29). Lateral root bases were embedded in 5% agar. Sections were analyzed under a stereomicroscope (Leica, Wetzlar, Germany). The differential staining was as described (30). Nonstained sections were observed with bright-field optics or epifluorescence (G-excitation, BP545 exciter filter) and an Olympus BH2 microscope (Olympus, Tokyo).
Transmission electron microscopy (TEM) and H2O2 localization were performed as described (18, 31). Electron probe x-rays were microanalyzed with a CM12 Scanning Transmission Electron Microscope equipped with a TRACOR TN 54 EDX spectrometer probe (Philips, Eindhoven, The Netherlands).
Pharmacology. Concentrations of inhibitors were 7 or 14 μM l-α-(2-aminoethoxyvinyl)-glycine (AVG), 0.1 mM (aminooxy)-acetic acid, 10 mM α-aminoisobutyric acid, 1 μMAg2SO4, 0.05% (vol/vol) 2,5-norbornadiene, 1 μM diphenyleneiodonium chloride (DPI), 200 μM diethyldithio carbamic acid, and 1 mM ascorbic acid. Inhibitors were purchased from Sigma-Aldrich, except for 2,5-norbornadiene (Avocado, La Tour du Pin, France). The media were refreshed regularly. All experiments were done at least twice with four plants per time point. Final concentrations of H2O2, ethylene, and 2-chloroethylphosphonic acid were 1 mM, 21 μl per tube, and 20 μM, respectively. A second dose of ethylene was applied after 5 days. Three independent experiments were done with 16 plants per treatment. At the concentrations used, the inhibitors had no influence on plant or bacterial growth.
Results
Intercellular Colonization at Lateral Root Bases Is Associated with Local Plant Cell Death. During lateral root base nodulation, bacteria contact outer cortical plant cells at cracks in the fissure of the epidermis and induce infection pockets, whereas nodule primordia develop in the mid-inner cortex (Fig. 1A). The size and shape of infection pockets correspond to one or several cortical cells (Fig. 1 A). Hydroponic roots were inoculated with ORS571(pBBR5-hem-gfp5-S65T) and stained 2 days later with Evans blue to detect dead plant cells. Sections showed a few Evans blue-stained cells, in the outer cortex of the fissure region that colocalized with bacteria marked with green-fluorescent proteins (Fig. 1 B and C).
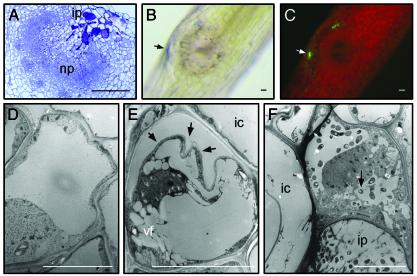
Fig. 1.
Plant cell death during infection pocket formation. (A) Toluidine blue-stained section through a 3-day-old developing root nodule. Evans blue-stained lateral root base, 2 days after inoculation with ORS571(pBBR5-hem-gfp5-S65T) viewed with bright-field optics (B) or epifluorescence (C). TEM of infection pocket region of stem-borne developing nodules 4 days after inoculation: healthy cortex cell (D), early stage of cell death (E) (arrows indicate disruption of wall–plasma membrane integrity), and dead plant cell at the edge of infection pocket (F) (arrow shows invaded bacteria). ic, inner cortex; ip, infection pocket; np, nodule primordium; vf, vacuole fragmentation. [Bars = 100 μm (A–C) and 10 μm (D–F).]
TEM on developing stem nodules revealed features of cell death in the invasion region (Fig. 1 D–F). Compared with healthy neighboring cells (Fig. 1D), dying cells had disrupted cell wall–plasma membrane integrity (Fig. 1E, arrows), vacuole fragmentation, and electron-dense precipitates in the cytoplasm (Fig. 1E); they lost rigidity and became invaded by bacteria (Fig. 1F, arrow).
Ethylene and H2O2 Mediate the Nod Factor Response and Nodulation. Hydroponic roots of S. rostrata carried only few root hairs, mainly on the oldest parts. Bulge-like structures were present at lateral root bases (Fig. 2A). On application of purified Nod factors, they grew out into bushes of distorted root hairs (Fig. 2B). No effects were caused by chitopentaose (data not shown), whereas exogenous ethylene, 2-chloroethylphosphonic acid (an ethylene-releasing compound), or 1-aminocyclopropane-1-carboxylate (the ethylene precursor) as well as H2O2, all triggered outgrowth of bushes of straight axillary root hairs (Fig. 2C; see Fig. 5, which is published as supporting information on the PNAS web site, www.pnas.org.). To test the hypothesis that Nod factors stimulate endogenous ethylene/ROS production, different agents that interfere with ethylene synthesis or perception, ROS generation (AVG, (aminooxy)acetic acid, α-aminoisobutyric acid, Ag+ ions, 2,5-norbornadiene, DPI, and diethyldithio carbamic acid), and the H2O2 scavenger ascorbic acid were used. When these agents were added 1 or 2 days before Nod factors, axillary root hair outgrowth was prevented. DPI could not prevent ethylene-induced axillary root hair growth, suggesting that ROS production may precede ethylene production during Nod factor-stimulated root hair growth (see Fig. 5).
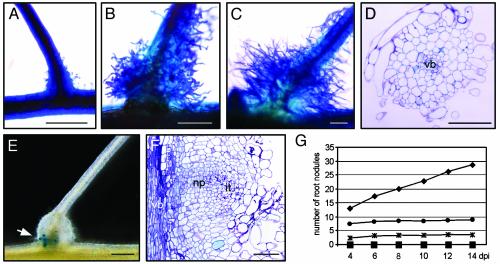
Fig. 2.
Ethylene and ROS involvement in Nod factor responses and nodulation. (A–C) Methylene blue-stained lateral root bases, observed 5 days postinoculation: control (A), treated with 10–9 M Nod factors (B), and with ethylene (C). (D) Transverse toluidine blue-stained section through a lateral root base inoculated with ORS571 after pretreatment with Ag+.(E) Root pretreated with 7 μM AVG, inoculated with ORS571(pRG960SD-32), 6 days after inoculation (arrow indicates bacteria viewed as blue GUS precipitate). (F) Toluidine blue-stained longitudinal section through E. (G) Influence of Ag+ on nodule initiation; roots were not treated (diamond) or were treated with Ag+ 2 days before (small square), 1 day before (triangle), simultaneously with (large square), 1 day after (asterisk), or 2 days after (circle) inoculation with ORS571. Note that the small square and triangle are masked by the large square. For abbreviations, see Fig. 1; vb, vascular bundle. [Bars = 100 μm (A–C and F) and 1 mm (D and E).]
Also, inhibitors of ethylene synthesis and perception, ROS production, and ascorbic acid blocked nodulation when they were added 2 days before the bacteria (data not shown). After hydroponic roots pretreated with AVG had been inoculated with a GUS-marked strain [ORS571(pRG960SD-32)], in 50–60% of the roots, lateral root bases were unaltered, no GUS staining was observed, and toluidine blue-stained sections looked identical to untreated, uninoculated controls (Fig. 2D); in 40–50% of the roots, lateral root bases were slightly swollen but did not enlarge with time and were always infected by bacteria (Fig. 2E; data not shown). Nodule primordia had been initiated and no infection pockets were seen in the outer cortex, but occasionally, small infection threads were formed (Fig. 2F). In experiments with Ag+ ions and α-aminoisobutyric acid, similar observations were made (data not shown). Thus, ethylene inhibitors either completely blocked invasion and nodule primordia, or small swellings were formed that had features of abortive infection and organ initiation.
To investigate whether ethylene was needed at later stages of nodule development, Ag+ ions were supplemented at different time points. When added 2 days or 1 day before, or simultaneously, with azorhizobia, Ag+ ions blocked nodulation completely (Fig. 2G). When added 1 day or 2 days after the bacteria, a limited number of functional nodules were formed, presumably corresponding to initiation events that had taken place before addition of the inhibitor. In control experiments, nodule numbers increased with time, illustrating an ongoing process of nodule initiation (Fig. 2G). An experiment with (aminooxy)acetic acid gave similar results (data not shown). Exogenous ethylene had no effect on nodulation of hydroponic roots of S. rostrata (see Fig. 6, which is published as supporting information on the PNAS web site).
Nod Factor- and Ethylene-Induced Changes in Cortical Tissues of Lateral Root Bases. In sections through lateral root bases of hydroponic control roots, a few cortical cells were strongly stained with toluidine blue (designated “blue cells” hereafter), and, occasionally, small spaces occurred between cortical cells (Fig. 3A, arrow). Ethylene treatment (Fig. 3 B–D) resulted in swelling of lateral root bases, with enlarged cells, an increased number of blue cells, and large cavities that contained remainders of cell walls (Fig. 3D, arrowhead). Treatment with Nod factors likewise caused large cavities and increased numbers of blue cells (Fig. 3E), but also foci of dividing cells appeared (Fig. 3 E and F) and, occasionally, small nodule primordia and a few vascular bundles (Fig. 3F). When AVG was added before Nod factors, most roots did not respond (70 and 90% of roots with 7 and 14 μM AVG, respectively; Fig. 3G) and sections looked similar to untreated, uninoculated controls (data not shown). A few lateral root bases were somewhat swollen (Fig. 3G), which was associated with limited cell division, cell enlargement, and some cavities (Fig. 3H). Similar results were obtained with Ag+ and DPI pretreatments (data not shown). Blue cells had features of cell death on differential staining (dark-blue nucleus; Fig. 3 I and J; ref. 30). In nonstained sections, they were brownish under bright-field (Fig. 3K) and fluorescent under epifluorescent microscopy (Fig. 3L). In a TEM analysis of Nod factor-treated lateral root bases, blue cells showed features of death (see Fig. 7, which is published as supporting information on the PNAS web site).
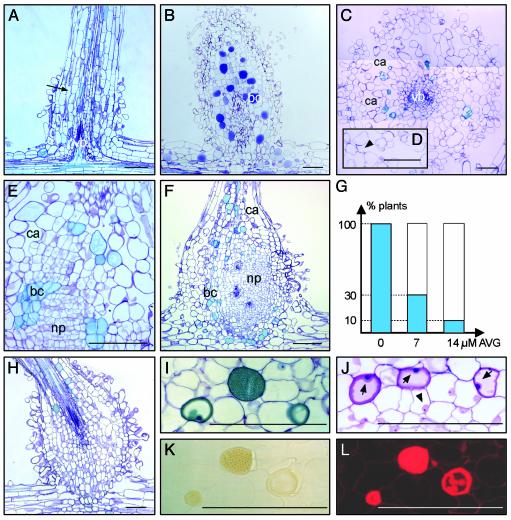
Fig. 3.
Nod factor and ethylene effects on lateral root bases. Roots were treated with 10–9 M Nod factors or ethylene for 5 days. All sections except J–L were stained with toluidine blue. (A) Mock-inoculated root. Arrow indicates intercellular spaces. (B and C) Ethylene treatment. (D) Detail of C; arrowhead indicates cavity with cell wall remainders. (E and F) Nod factor treatment. (G) Percentages of plants that show lateral root base enlargements on Nod factor application after pretreatment with AVG. (H) Example of a Nod factor-induced swelling after pretreatment with AVG. (I) Toluidine blue-stained section through a Nod factor-treated lateral root base. (J) Differential staining of analogous section of I. Dark-blue nuclei (arrows) indicate cells are dying; arrowhead marks nucleus of a healthy cell. (K) Unstained neighboring section of I under bright-field optics. (L) Epifluorescence microscopy of K. For abbreviations, see Fig. 1; bc, blue cell; ca, cavity. (Bars = 100 μm.)
H2O2 Colocalizes with Plant Cell Death During Bacterial Invasion. To localize H2O2, TEM was used to visualize electron-dense precipitates of cerium perhydroxides caused by the reaction of H2O2 with CeCl3 (31). Adventitious rootlets were harvested 50 and 70 h post inoculation (hpi) with ORS571. At 50 hpi, H2O2 was detected in the plasma membrane/cell wall of a few outer cortical cells of the fissure region. One cell was surrounded by H2O2 and showed vacuolar fragmentation and loss of shape and rigidity (Fig. 4A). No H2O2 was detected in the nodule primordia (data not shown).
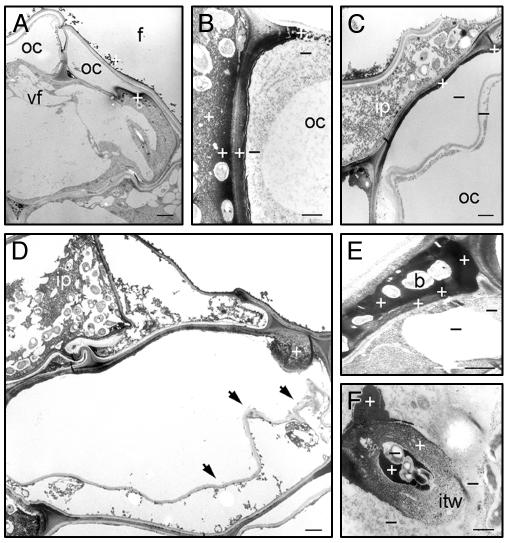
Fig. 4.
H2O2 localization during nodule initiation. TEM sections of CeCl3-stained adventitious rootlets, harvested at 50 (A) and 70 (B–F) hpi with ORS571. Plus or minus indicates presence or absence of Ce perhydroxides, confirmed by electron probe x-ray microanalyses. (A) Dying outer cortex cell. (B) Infection pocket-cortical cell boundary. (C) Outer cortex cell neighboring infection pocket. (D) Papillus-forming dying outer cortex cell; arrows indicate disruption of wall-plasma membrane integrity. (E) Intercellular infection thread. (F) Intracellular infection thread. For abbreviations, see Fig. 1; b, bacteria; cw, cell wall; f, fissure; itw, infection thread wall. (Bars = 1 μm.)
At 70 hpi, massive amounts of H2O2 were found in parts of walls and plasma membranes of plant cells in the vicinity of bacteria in infection pockets and the matrix of infection pockets (Fig. 4B). Hardly any H2O2 was detected in bacteria, which were surrounded by a thick low electron-dense capsule (Fig. 4 B–D). At sites where high amounts of H2O2 were present, the cell wall–plasma membrane integrity was disrupted (Fig. 4D). Severe features of cell death were observed, such as chromatin condensation and degeneration of the nucleus (data not shown). H2O2-containing papillae were present in parts of cell walls in contact with bacteria or dying cells (Fig. 4D). H2O2 was also detected in the matrix and walls of intercellular and of some intracellular infection threads (Fig. 4 E and F).
Discussion
Nodulation at the bases of lateral roots or adventitious rootlets of S. rostrata depends on intercellular invasion, as opposed to root hair colonization and intracellular entry. A TEM study has shown that plant cells surrounding invading bacteria have features that are reminiscent of cell death during development and responses to biotic or environmental stresses (32). Staining with Evans blue, in combination with visualization of bacteria marked by green-fluorescent proteins, confirmed that bacterial colonization is accompanied with local cell death. Because infection pocket formation depends on Nod factors, the question arose whether Nod factors trigger endogenous plant signals that could mediate cell death.
A clue to secondary signals came from pharmacological studies, monitoring the Nod factor-induced outgrowth of axillary root hairs. This response was not elicited by chitin oligomers and displayed the same dependency on specific Nod factor modifications as nodule initiation (27). Inhibitors of ethylene synthesis and perception, inhibitors of ROS generation, and a H2O2 scavenger, all blocked the Nod factor-induced root hair formation, indicating that endogenous ethylene and ROS mediate the response. Accordingly, axillary root hair growth on hydroponic roots of S. rostrata was induced by exogenous ethylene and H2O2. Also in Arabidopsis thaliana, ethylene plays a role in root hair growth (33), and changes in the redox status of the environment may influence induction of root hair elongation (34).
That Nod factors may stimulate ethylene and ROS synthesis has been observed in a few other legumes. In Medicago truncatula, Nod factors trigger production of superoxide radicals in a zone close to the root tip (17). Vicia sativa roots develop “thick and short roots” (Tsr) on inoculation in the light with Rhizobium leguminosarum bv. viciae (35). The Tsr phenotype was mediated by an excess of ethylene (35) and could be triggered by purified Nod factors (12), suggesting that Nod factors cause a local ethylene production, which, in light-grown roots, leads to high ethylene levels and a Tsr phenotype (12).
Interestingly, endogenous ethylene and ROS synthesis were also required for initiation of nodulation on hydroponic roots of S. rostrata. In the presence of ethylene inhibitors, there were no signs of bacterial invasion, infection pocket formation, or nodule primordium formation. Occasional swellings, the likely consequence of leaky inhibition, showed both aborted bacterial invasions and arrested nodule primordia. Because invasion and division could not be uncoupled from ethylene or ROS requirement, they must be part of coordinated processes. Once nodule initiation had taken place, nodule development and function did not depend on ethylene. Ethylene requirement is a special feature of S. rostrata nodulation. In Medicago sativa or Glycine max, nodulation was promoted or not influenced by ethylene inhibitors, respectively (8, 9, 36). Molecular data have confirmed the ethylene requirement: clones homologous to genes coding for S-adenosyl-l-methionine synthetases, 1-aminocyclopropane-1-carboxylate synthases and oxidases, which are three enzymes involved in ethylene synthesis, are up-regulated during intercellular invasion (unpublished data).
Nod factor treatment of hydroponic roots triggered cells to divide at lateral root bases, cavities to be formed with remainders of cell walls, and an increase in the numbers of cells with death features and accumulation of phenolic compounds. The latter two characteristics were equally induced by ethylene. Cells with related death symptoms occurred in the inner integument during seed coat development in Arabidopsis thaliana (37). In S. rostrata such cells could fortify aerenchymatic tissues as part of the water stress response. The cavities are indeed reminiscent of aerenchyma formation in hypoxic maize roots, a process in which ethylene plays a key role (38). Surprisingly, ethylene mediated both Nod factor-triggered lesion formation and division. Although in terrestrial plants, ethylene is involved in growth arrest and maturation, in semiaquatic plants it stimulates growth and division processes during submergence responses (21, 39, 40). In flooded Rumex palustris roots, ethylene increases auxin sensitivity, which may lead to cell division (41); in deepwater rice, ethylene is the main trigger of cell division during submergence-induced adventitious root growth (21). S. rostrata may have adopted similar strategies for water tolerance and recruited ethylene to mediate Nod factor responses for nodulation at lateral and adventitious root bases.
Pharmacological experiments showed that, in S. rostrata, ROS generation is important for nodule initiation. With the CeCl3 precipitation method, H2O2 was found to colocalize with different stages of invasion at adventitious root bases, but could not be detected in nodule primordia. H2O2 was first produced in the plasma membrane/wall of outer cortical cells in contact with bacteria. Later, plant cells flanking infection pockets were surrounded by high amounts of H2O2 and had features of cell death. Thus, ethylene and H2O2 may act together to promote cell death, perhaps reinforcing each other. ROS generation can, depending on the dosage, promote a local programmed cell death (32) and induce defense gene expression (7, 42). Cell death could be restricted by expression of genes coding for H2O2-scavenging functions; for instance, a peroxidase-encoding gene is induced in cells surrounding infection pockets (43).
The matrix and wall of intercellular and some intracellular infection threads contain high amounts of H2O2; the latter has also been observed in alfalfa nodules (44). Perhaps H2O2, as a substrate for cross-linking of macromolecules, contributes to wall rigidity and to infection thread growth (16). H2O2 in the matrix does not seem to harm the bacteria, probably because rhizobia produce protective enzymes (44) and are shielded by a capsule of (presumably) extracellular polysaccharides.
Clearly, H2O2 may play a critical role in multiple aspects of infection and nodule development (refs. 15 and 17 and this work). In S. rostrata, initiation of primordia depends on ROS, and the question arises whether this could be a general feature of legume nodulation. In animal systems, low concentrations of H2O2 are a potential mitogenic factor (45–48). Thus, we should also investigate whether a critical concentration of ROS could be a signal for division into deeper cortical layers in legumes.
Bacterial colonization with infection pocket formation is not unique for S. rostrata. In legumes, intercellular invasion has been described during nodulation of Stylosanthes sp., Neptunia sp., Mimosa scabrella, Aeschenomyne afraspera, and Chamaecytisus proliferus (49–54). The diazotrophic endophyte Azoarcus forms colonies in the aerenchyma of flood-tolerant Kallar grass (55, 56). Intercellular colonies are formed during root invasion by pathogenic Ralstonia solanacearum (57) and in the upper parts of host plants by the biotrophic phytopathogen, Rhodococcus fascians (58). Our data provide a basis for future investigations of the differences and similarities in pathogenic and beneficial intercellular colonization strategies, as a way to understand the subtle edge between symbiosis and pathogenicity.
Supplementary Material
Acknowledgments
We thank Ghislaine Van De Sype, Didier Hérouart, and Alain Puppo (Université de Nice-Sophia Antipolis) for help with electron probe x-ray microanalyses, Gilbert Engler (Ghent University) and Maurice De Proft (Catholic University Leuven, Leuven, Belgium) for helpful suggestions and discussions, Stefan Eberhard (University of Georgia, Athens) for help with microscopy picturing, and Martine De Cock and Karel Spruyt for preparing the manuscript. This work was supported by grants from the Interuniversity Poles of Attraction Programme (Belgian State, Prime Minister's Office, Federal Office for Scientific, Technical and Cultural Affairs; P5/13), the Human Potential and Mobility Research Training Network (HPRN-CT-2000-00094), and Tournesol. W.D. and W.C. are indebted to the Vlaamse Wetenschappelijke Stichting and the Instituut voor de aanmoediging van Innovatie door Wetenschap en Technologie in Vlaanderen for a travel grant and predoctoral fellowship, respectively.
Abbreviations: AVG, l-α-(2-aminoethoxyvinyl)-glycine; DPI, diphenyleneiodonium chloride; GUS, β-glucuronidase; hpi, hours postinoculation; ROS, reactive oxygen species; TEM, transmission electron microscopy.
References
- 1.Sprent, J. I. (2002) Nodulation in Legumes (Royal Botanical Gardens, Kew, U.K.).
- 2.Perret, X., Staehelin, C. & Broughton, W. J. (2000) Microbiol. Mol. Biol. Rev. 64, 180–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kistner, C. & Parniske, M. (2002) Trends Plant Sci. 7, 511–518. [DOI] [PubMed] [Google Scholar]
- 4.D'Haeze, W. & Holsters, M. (2002) Glycobiology 12, 79R–105R. [DOI] [PubMed] [Google Scholar]
- 5.Parniske, M. (2000) Curr. Opin. Plant Biol. 3, 320–328. [DOI] [PubMed] [Google Scholar]
- 6.Wang, K. L.-C., Li, H. & Ecker, J. R. (2002) Plant Cell 14, Suppl., S131–S151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Delledonne, M., Murgia, I., Ederle, D., Sbicego, P. F., Biondani, A., Polverari, A. & Lamb, C. (2002) Plant Physiol. Biochem. 40, 605–610. [Google Scholar]
- 8.Peters, N. K. & Crist-Estes, D. K. (1989) Plant Physiol. 91, 690–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Penmetsa, R. V. & Cook, D. R. (1997) Science 275, 527–530. [DOI] [PubMed] [Google Scholar]
- 10.Lee, K. H. & LaRue, T. A. (1992) Plant Physiol. 100, 1759–1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guinel, F. C. & Sloetjes, L. L. (2000) J. Exp. Bot. 51, 885–894. [PubMed] [Google Scholar]
- 12.van Spronsen, P. C., van Brussel, A. A. N. & Kijne, J. W. (1995) Eur. J. Cell Biol. 68, 463–469. [PubMed] [Google Scholar]
- 13.Oldroyd, G. E. D., Engstrom, E. M. & Long, S. R. (2001) Plant Cell 13, 1835–1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heidstra, R., Yang, W. C., Yalcin, Y., Peck, S., Emons, A., van Kammen, A. & Bisseling, T. (1997) Development 124, 1781–1787. [DOI] [PubMed] [Google Scholar]
- 15.Santos, R., Hérouart, D., Sigaud, S., Touati, D. & Puppo, A. (2001) Mol. Plant-Microbe Interact. 14, 86–89. [DOI] [PubMed] [Google Scholar]
- 16.Wisniewski, J.-P., Rathbun, E. A., Knox, J. P. & Brewin, N. J. (2000) Mol. Plant-Microbe Interact. 13, 413–420. [DOI] [PubMed] [Google Scholar]
- 17.Ramu, S. K., Peng, H.-M. & Cook, D. R. (2002) Mol. Plant-Microbe Interact. 15, 522–528. [DOI] [PubMed] [Google Scholar]
- 18.D'Haeze, W., Gao, M., De Rycke, R., Van Montagu, M., Engler, G. & Holsters, M. (1998) Mol. Plant-Microbe Interact. 11, 999–1008. [Google Scholar]
- 19.Duhoux, E. (1984) Can. J. Bot. 62, 982–994. [Google Scholar]
- 20.Ndoye, I., de Billy, F., Vasse, J., Dreyfus, B. & Truchet, G. (1994) J. Bacteriol. 176, 1060–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lorbiecke, R. & Sauter, M. (1999) Plant Physiol. 119, 21–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fernández-López, M., Goormachtig, S., Gao, M., D'Haeze, W., Van Montagu, M. & Holsters, M. (1998) Proc. Natl. Acad. Sci. USA 95, 12724–12728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dreyfus, B., Garcia, J. L. & Gillis, M. (1988) Int. J. Syst. Bacteriol. 38, 89–98. [Google Scholar]
- 24.Van den Eede, G., Dreyfus, B., Goethals, K., Van Montagu, M. & Holsters, M. (1987) Mol. Gen. Genet. 206, 291–299. [Google Scholar]
- 25.Van den Eede, G., Deblaere, R., Goethals, K., Van Montagu, M. & Holsters, M. (1992) Mol. Plant-Microbe Interact. 5, 228–234. [DOI] [PubMed] [Google Scholar]
- 26.Goethals, K., Gao, M., Tomekpe, K., Van Montagu, M. & Holsters, M. (1989) Mol. Gen. Genet. 219, 289–298. [DOI] [PubMed] [Google Scholar]
- 27.D'Haeze, W., Mergaert, P., Promé, J.-C. & Holsters, M. (2000) J. Biol. Chem. 275, 15676–15684. [DOI] [PubMed] [Google Scholar]
- 28.Mergaert, P., Ferro, M., D'Haeze, W., Van Montagu, M., Holsters, M. & Promé, J.-C. (1997) Mol. Plant-Microbe Interact. 10, 683–687. [DOI] [PubMed] [Google Scholar]
- 29.Mergemann, H. & Sauter, M. (2000) Plant Physiol. 124, 609–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kosslak, R. M., Chamberlin, M. A., Palmer, R. G. & Bowen, B. A. (1997) Plant J. 11, 729–745. [DOI] [PubMed] [Google Scholar]
- 31.Bestwick, C. S., Brown, I. R., Bennett, M. H. R. & Mansfield, J. W. (1997) Plant Cell 9, 209–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jones, A. M. (2001) Plant Physiol. 125, 94–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dolan, L. (2001) Curr. Opin. Plant Biol. 4, 550–554. [DOI] [PubMed] [Google Scholar]
- 34.Sánchez-Fernández, R., Fricker, M., Corben, L. B., White, N. S., Sheard, N., Leaver, C. J., Van Montagu, M., Inzé, D. & May, M. J. (1997) Proc. Natl. Acad. Sci. USA 94, 2745–2750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zaat, S. A. J., Van Brussel, A. A. N., Tak, T., Lugtenberg, B. J. J. & Kijne, J. W. (1989) Planta 177, 141–150. [DOI] [PubMed] [Google Scholar]
- 36.Schmidt, J. S., Harper, J. E., Hoffman, T. K. & Bent, A. F. (1999) Plant Physiol. 119, 951–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beeckman, T., De Rycke, R., Viane, R. & Inzé, D. (2000) J. Plant Res. 113, 139–148. [Google Scholar]
- 38.Drew, M. C., He, C.-J. & Morgan, P. W. (2000) Trends Plant Sci. 5, 123–127. [DOI] [PubMed] [Google Scholar]
- 39.Kende, H., van der Knaap, E. & Cho, H.-T. (1998) Plant Physiol. 118, 1105–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Voesenek, L. A. C. J., Benschop, J. J., Bou, J., Cox, M. C. H., Groeneveld, H. W., Millenaar, F. F., Vreeburg, R. A. M. & Peeters, A. J. M. (2003) Ann. Bot. 91, 205–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Visser, E. J. W., Cohen, J. D., Barendse, G. W. M., Blom, C. W. P. M. & Voesenek, L. A. C. J. (1996) Plant Physiol. 112, 1687–1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Levine, A., Tenhaken, R., Dixon, R. & Lamb, C. (1994) Cell 79, 583–593. [DOI] [PubMed] [Google Scholar]
- 43.Lievens, S. (2001) Ph.D. thesis (Ghent University, Ghent, Belgium).
- 44.Santos, R., Hérouart, D., Puppo, A. & Touati, D. (2000) Mol. Microbiol. 38, 750–759. [DOI] [PubMed] [Google Scholar]
- 45.Suh, Y.-A., Arnold, R. S., Lassegue, B., Shi, J., Xu, X., Sorescu, D., Chung, A. B., Griendling, K. K. & Lambeth, J. D. (1999) Nature 401, 79–82. [DOI] [PubMed] [Google Scholar]
- 46.Arnold, R. S., Shi, J., Murad, E., Whalen, A. M., Sun, C. Q., Polavarapu, R., Parthasarathy, S., Petros, J. A. & Lambeth, J. D. (2001) Proc. Natl. Acad. Sci. USA 98, 5550–5555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Preston, T. J., Muller, W. J. & Singh, G. (2001) J. Biol. Chem. 276, 9558–9564. [DOI] [PubMed] [Google Scholar]
- 48.Arbiser, J. L., Petros, J., Klafter, R., Govindajaran, B., McLaughlin, E. R., Brown, L. F., Cohen, C., Moses, M., Kilroy, S., Arnold, R. S., et al. (2002) Proc. Natl. Acad. Sci. USA 99, 715–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chandler, M. R., Date, R. A. & Roughley, R. J. (1982) J. Exp. Bot. 33, 47–57. [Google Scholar]
- 50.de Faria, S. M., Hay, G. T. & Sprent, J. I. (1988) J. Gen. Microbiol. 134, 2291–2296. [Google Scholar]
- 51.Alazard, D. & Duhoux, E. (1990) J. Exp. Bot. 41, 1199–1206. [Google Scholar]
- 52.Vega-Hernández, M. C., Pérez-Galdona, R., Dazzo, F. B., Jarabo-Lorenzo, A., Alfayate, M. C. & León-Barrios, M. (2001) New Phytol. 150, 707–721. [Google Scholar]
- 53.James, E. K., Sprent, J. I., Sutherland, J. M., McInroy, S. G. & Minchin, F. R. (1992) Ann. Bot. 69, 173–180. [Google Scholar]
- 54.Subba-Rao, N. S., Mateos, P. F., Baker, D., Pankratz, H. S., Palma, J., Dazzo, F. B. & Sprent, J. I. (1995) Planta 196, 311–320. [Google Scholar]
- 55.Reinhold-Hurek, B. & Hurek, T. (1998) Crit. Rev. Plant Sci. 17, 29–54. [Google Scholar]
- 56.Egener, T., Hurek, T. & Reinhold-Hurek, B. (1999) Mol. Plant-Microbe Interact. 12, 813–819. [DOI] [PubMed] [Google Scholar]
- 57.Vasse, J., Genin, S., Frey, P., Boucher, C. & Brito, B. (2000) Mol. Plant-Microbe Interact. 13, 259–267. [DOI] [PubMed] [Google Scholar]
- 58.Cornelis, K., Ritsema, T., Nijsse, J., Holsters, M., Goethals, K. & Jaziri, M. (2001) Mol. Plant-Microbe Interact. 14, 599–608. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.