Abstract
Auxin redistribution along gravistimulated maize coleoptiles causes differential expression of the auxin-induced K+-channel gene ZMK1 (Zea mays K+ channel 1) and precedes the curvature response. To evaluate the role of ZMK1 during phototropism, we here investigated blue light-stimulated coleoptiles. Four hours of blue light stimulation resulted in phototropic bending (23°). Rotation on a clinostat, at nominally “zero” gravity, and simultaneous stimulation with unidirectional blue light, however, resulted in up to 51° bending toward the light. Differential ZMK1 transcription reached a maximum after 90 min of blue light stimulation under gravity, whereas ZMK1 expression remained asymmetric for at least 180 min in photostimulated coleoptiles on a clinostat. We therefore conclude that the stronger phototropic bending under nominally “zero” gravity results from prolonged differential expression of ZMK1. Under both conditions, asymmetric expression of ZMK1 could be superimposed on the lateral auxin gradient across the coleoptile tip, whereas the gene for the blue light receptor phototropin 1 (PHOT1), expressed in the tip only, was not differentially regulated in response to blue light. The activation of the two different receptors eliciting the photo- and gravitropic response of the coleoptile thus feeds into a common signaling pathway, resulting in auxin redistribution in the coleoptile tip and finally in differential transcription of ZMK1. In the process of signal integration, gravity transduction restricts the magnitude of the blue light-inducible ZMK1 gradient. The spatial and temporal distribution of ZMK1 transcripts and thus differential K+ uptake in both flanks of the coleoptile seem to limit the stimulus-induced bending of this sensory organ.
Studying the tropistic curvature of plant organs, Charles Darwin (1) postulated the existence of a signal molecule being synthesized in the tip of photostimulated grass coleoptiles and enhancing growth in one flank of the organ. Went (2) and Cholodny (3) could show that this substance, called auxin, is laterally translocated in photostimulated coleoptiles, resulting in a curvature of the plant toward the light source. Using radioactively labeled auxin, Parker and Briggs (4) and Iino (5) confirmed the Cholodny–Went hypothesis (6). Whereas lateral auxin translocation in photostimulated maize seedlings is restricted to the tip (7), auxin redistribution in gravistimulated seedlings occurs along the length of the entire coleoptile (8). Tropistic curvature is preceded by the asymmetric distribution of apoplastic protons (9), the drop in extracellular pH being caused by increased indole-3-acetic acid (IAA) levels in the fastergrowing flank of the organ (for the Acid Growth Theory, see ref. 10). Increased proton extrusion results from auxin activation of the plasma membrane H+ ATPase and in the long-term rise in the number of H+ pumps (refs. 11–13; for a review, see ref. 14). Acidification of the apoplast activates expansins mediating cell wall loosening, a prerequisite for cell expansion (for a review, see ref. 15).
Although the incoming signal is transduced with the help of one single phytohormone, signal perception is different in graviand photostimulated plants. The light signal is perceived by photoreceptor proteins (phototropins). In Arabidopsis thaliana, two phototropins, phot1 and -2, act together in perceiving the blue-light stimulus (16) and evoke blue-light induced cytoplasmic Ca2+ signals (17), most likely via calcium-permeable channels (18). An ortholog to phototropin 1 (PHOT1) has already been isolated from maize (GenBank accession no. AF033263; for a review, see ref. 19). Interestingly, the blue light-responsive protein in maize has been shown to be most abundant in the apical 5-mm-tip zone of coleoptiles (20), a localization in line with the photoperceptivity of this organ (21).
Gravity sensing most likely results from the translocation of statholiths (22). The molecular mechanism of graviperception, however, remains still unknown. In A. thaliana, the isolation of auxin-sensitivity mutants led to the identification of components of the auxin influx (AUX family; ref. 23) and efflux carrier (PIN family; refs. 24 and 25). The agravitropic nature of these mutants, the polar localization of the respective protein (26), and its redistribution on gravistimulation (27) point to their role in polar and lateral auxin transport (for a review, see ref. 28). Moreover, a putative auxin receptor, ABP1, has been crystallized with 1-naphthylacetic acid (29) and mediates auxin-induced cell expansion (30). ABP1 deletion results in embryo lethality (31).
Thus, two physically distinct stimuli, blue light and gravity, merge into one common signaling pathway, leading to the redistribution of endogenous auxin and differential growth in the two flanks of the maize coleoptile. In a search for the osmotic motor involved in auxin-induced growth, potassium-dependent steps were identified and control of the osmotic motor by K+ channels was predicted (32). In a previous study, we isolated the voltage-dependent inwardly rectifying K+ channel ZMK1 (Zea mays K+ channel 1) from maize coleoptiles (8). ZMK1 transcription is induced by active auxins, and gravistimulation results in a differential expression in the two flanks of the graviresponding coleoptile. Because coleoptile elongation and bending involve transcriptional activation of ZMK1 and in turn increase in K+-channel protein density (33), this K+ channel seems to represent an essential element of the osmotic motor required for gravitropic curvature.
To explore whether differential ZMK1 expression is involved in the phototropic curvature of maize coleoptiles as well, we followed the redistribution of ZMK1 transcripts in photostimulated plants in the presence of gravity or at nominally “zero” gravity. We demonstrated that differential expression of ZMK1 during phototropism is coupled to the redistribution of auxin in the coleoptile tip. Furthermore, the extent of the curvature response of maize seedlings and the degree of differential ZMK1 expression were closely correlated. Thus, we conclude, that ZMK1 is involved in both gravitropic and phototropic bending of maize coleoptiles.
Materials and Methods
Maize Strains and Growth Conditions. Quantitative real-time RTPCR experiments were performed on tissues from Z. mays seedlings (hybrid corn cv. “Oural”). Maize caryopses were soaked in water, germinated on moistened filter paper, and grown under red light (0.2 μmol·m–2·s–1) at 25°C, because red light treatment is necessary for maize coleoptiles to acquire responsiveness for time-dependent phototropism (34). After 72 hr, the seedlings were transferred into pots filled with 1% agar. For phototropic stimulation, the apical 5 mm of the 4- to 5-day-old and 2- to 2.5-cm-long seedlings were illuminated with unilateral blue light (450 nm, 10 μmol·m–2·s–1). The plane passing through the two vascular bundles of the coleoptile was parallel to the direction of the light. Alternatively, plants were placed horizontally onto a clinostat [“Blue Light Cycler” (BLC); see below] rotating at 1 rpm (0.0001 × g). Stimulation with unilateral blue light started 30 min after onset of rotation. Coleoptiles were harvested after given time intervals. All treatments took place under continuous red light. For quantification of coleoptile curvature, seedlings were taken out of the pots 4 hr after the onset of blue light or gravity stimulation (compare ref. 8), attached to a strip of adhesive tape, and fixed to a Plexiglas plate. The plate was photocopied, and the curvature angle between the tangents drawn at the base and the top of the coleoptile was determined.
BLC. To uncouple the phototropic response of coleoptiles from gravity, we constructed a clinostat allowing illumination of seedlings with unilateral blue light during continuous rotation (see Fig. 5a, which is published as supporting information on the PNAS web site, www.pnas.org). Thus, we named this clinostat BLC. The clinostat is driven by a 12-V motor (Mabuchi Motor, Frankfurt, RS 380/385). The gear transmission ratio is 1:8,640, resulting in one rotation per minute. Previous experiments have shown that this speed is sufficient to prevent the seedlings from resolving the direction of the omnilaterally occurring gravistimulus. Therefore, seedlings rotated on the BLC for 24 h without blue light treatment showed random curvature with respect to the 360° possible (see Fig. 5b Right). In every slot, the individual seedling is facing a blue light-emitting diode (Nichia Europe Bv., Amsterdam, NLPB 500, 450 nm). Light intensity was adjusted to 10 μmol·m–2·s–1.
Quantitative Real-Time RT-PCR. Isolation of RNA from maize coleoptiles, quantitative real-time RT-PCR (LightCycler, Roche Diagnostics), and quantification of transcripts were performed as described in ref. 35. The following ZMK1- and PHOT1-specific primers were used: ZMK1 fw (5′-ataacaatgggcatacag-3′), ZMK1 rev (5′-ttccgtctttcattgag-3′), PHOT1 fw (5′-ccacggtcattgtcag-3′), and PHOT1 rev (5′-ctt-cccttggttttagaatg-3′). All quantifications were normalized to the signal of actin cDNA fragments generated by the primers ZmAct 81/83 fw (5′-acacagtgccaatct-3′) and ZmAct 81/83 rev (5′-actgagcacaatgttac-3′).
Analysis of Endogenous IAA Concentrations. Coleoptiles of 2 cm in length were harvested after phototropic stimulation as described in ref. 8. Purification and quantification of endogenous concentrations of IAA were done on triplicate samples by GC-MS as described (8, 36).
Results
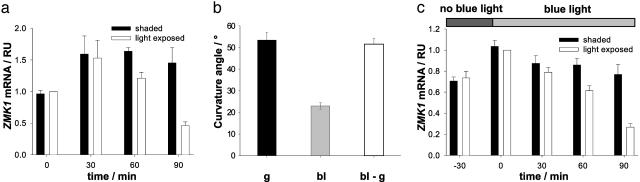
ZMK1 Expression During Phototropic Curvature of the Maize Coleoptile. In previous experiments, it could be shown that, during gravitropic bending of maize coleoptiles, the transcriptional induction of the K+-channel gene, ZMK1, follows the redistribution of auxin (8). To determine whether ZMK1 is transcriptionally regulated during phototropic growth as well, maize seedlings were illuminated with unilateral blue light to stimulate phototropic bending. In parallel, we monitored the ZMK1 mRNA content in the illuminated and shaded half of the coleoptile by quantitative real-time RT-PCR over a period of 90 min (Fig. 1a). Thirty minutes after the onset of blue light stimulation, ZMK1 transcripts increased on both sides of the coleoptile. During prolonged stimulation, ZMK1 transcripts decreased in the light-exposed side, which is growth restricted and auxin depleted (compare Fig. 2b), whereas they remained elevated in the growing shaded half of the coleoptile. As a result of an ongoing differential expression of the ZMK1 gene in response to photostimulation, the level of ZMK1 mRNA was up to 3-fold higher in the shaded than in the illuminated side of the coleoptile 90 min after stimulus onset. These results indicate that differential expression of the K+-uptake-channel gene ZMK1 is involved in phototropic as well as gravitropic curvature of maize coleoptiles (compare ref. 8). Although differential expression of ZMK1, detectable 60 min after photostimulation, cannot account for the initial steps of the phototropic response, which can be observed already 20–30 min after the onset of blue light stimulation (37), it might be essential for sustained curvature growth (see Discussion).
Fig. 1.
Differential expression of ZMK1 and coleoptile bending angles during gravi- and phototropism of the maize coleoptile. (a) Relative transcript level (RU, relative units) of ZMK1 in light-exposed (open bars) and shaded (solid bars) coleoptile halves after 0, 30, 60, and 90 min of stimulation with unilateral blue light. The mRNA content (n = 3 ± SE) was quantified as described in Materials and Methods. The level of ZMK1 mRNA in the light-exposed half at t = 0 min was set to 1.0. (b) Curvature angle of coleoptiles after 4 hr of gravistimulation (g, black bar) and stimulation with unilateral blue light (bl, gray bar) and of photostimulated seedlings rotating on the BLC (bl-g, open bar). Bending angles (mean of n ≥ 30 ± SE) were measured as described in Materials and Methods. (c) Relative transcript level (RU) of ZMK1 in light-exposed (open bars) and shaded (closed bars) coleoptile halves of seedlings rotating on the BLC after 0, 30, 60, and 90 min of simultaneous stimulation with unilateral blue light. Blue light illumination started 30 min after the onset of clinostat rotation (–30). The mRNA content (n = 3 ± SE) was quantified as described in Materials and Methods. The level of ZMK1 mRNA in the light-exposed half at t = 0 min was set to 1.0.
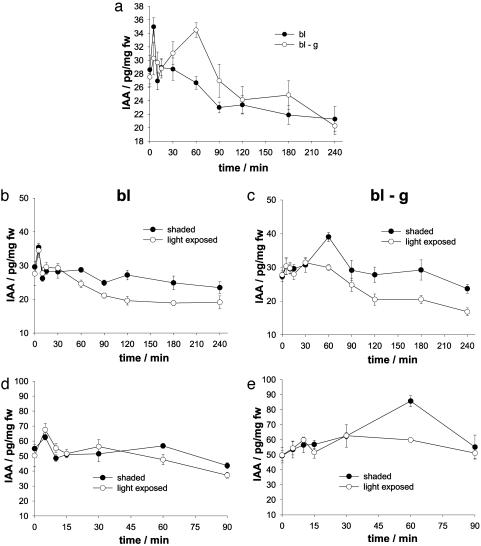
Fig. 2.
Redistribution of endogenous IAA in response to stimulation of the coleoptile with blue light. (a) IAA concentration in the entire coleoptile, stimulated with unilateral blue light under normal gravity conditions (bl, filled circles) and on the rotating BLC (bl-g, open circles). (b) IAA concentration in shaded (filled circles) and light-exposed (open circles) coleoptile halves after photostimulation (bl). (c) IAA concentration in shaded (filled circles) and light-exposed (open circles) coleoptile halves after photostimulation on the rotating BLC (bl-g). (d) IAA concentration in shaded (filled circles) and light-exposed (open circles) sides of the coleoptile tip after photostimulation (bl). (e) IAA concentration in shaded (filled circles) and light-exposed (open circles) sides of the coleoptile tip after photostimulation on the rotating BLC (bl-g). Stimulation with blue light occurred for 240 min (a–c) or 90 min (d and e). IAA concentrations are given in picograms of IAA/milligram of fresh weight (fw), mean ± SE, n = 3.
To further compare the phototropic and gravitropic curvature responses of coleoptiles, we determined the bending angles in response to both stimuli (Fig. 1b). Gravistimulated maize seedlings reached an average bending angle of 53.4 ± 3.5° (n = 32, mean ± SE) within 4 hr after displacement into a horizontal position. In coleoptiles stimulated with unilateral blue light, the final curvature after 4 hr was much less pronounced (average bending angle of only 23 ± 1.4°, n = 30, mean ± SE). Thus, gravitropism may counteract phototropic bending because the blue light stimulates the seedlings to bend away from the vertical axis. To test this hypothesis, we rotated maize seedlings on a clinostat and illuminated the tips of the coleoptiles with unilateral blue light (BLC; see Materials and Methods and Fig. 5). Under these conditions, the bending angle 4 hr after the onset of photostimulation was the same as for the gravistimulated plants, i.e., 51.5 ± 2.7° (n = 32, mean ± SE, Fig. 1b). It was thus tempting to speculate that the stronger curvature of the coleoptile, when uncoupled from gravity, would result from a more pronounced difference in ZMK1 expression on both sides of the coleoptile. As shown by Philippar et al. (8), ZMK1 expression after 90 min of gravistimulation is up to six times higher in the faster-growing lower side than in the growth-restricted upper side of maize coleoptiles, leading to a final curvature of ≈55° (Fig. 1b), whereas photostimulated plants displayed a much smaller bending angle and a less pronounced gradient in ZMK1 expression (3:1, Fig. 1a).
To explore whether the ZMK1 mRNA gradient is steeper in photostimulated plants rotated on a clinostat, we applied the following protocol: after 30-min rotation of the seedlings on the BLC, blue light stimulation was initiated and the ZMK1 mRNA content in the light-exposed and in the shaded half of the coleoptile was monitored by quantitative real-time RT-PCR over a period of 90 min (Fig. 1c). Although rotation on the clinostat for 30 min without blue light stimulation evoked a symmetric increase of ZMK1 mRNA in the coleoptile, ZMK1 transcripts after the onset of blue light stimulation continuously decreased in the growth-restricted, light-exposed side. In the shaded half, which is characterized by rapid growth, ZMK1 transcription remained constant, leading to a 3-fold higher ZMK1 expression when compared with the illuminated half after 90 min of blue light treatment. This shows that, although the curvature of the coleoptiles uncoupled from gravity was 2-fold stronger, the gradient of ZMK1 transcripts in the coleoptile halves remained the same when compared with the phototropic curvature under normal gravity (compare Fig. 1).
Auxin Redistribution During Phototropic Curvature. Because auxin redistribution in the coleoptile precedes both gravi- and phototropic bending (5), we wanted to explore whether the IAA concentration in photostimulated coleoptiles is modified under different gravity conditions. Therefore, we measured the auxin content at given time points in whole coleoptiles stimulated with unilateral blue light under normal gravity and on the BLC (Fig. 2a). The initial auxin concentration was 28 ± 0.5 pg/mg fresh weight (fw) under both conditions. Within 15 min after the onset of blue light stimulation, the auxin concentration peaked, whereas this initial maximum (after 5 min) was much more pronounced in plants photostimulated under normal gravity. In coleoptiles exposed to blue light under normal gravity, the IAA concentration further decreased during the time course of the experiment, reaching a basal level of 23 ± 0.8 pg/mg fw 90 min after stimulus onset. In contrast, levels of free IAA increased significantly in plants photostimulated on the rotating BLC, reaching a maximal IAA concentration of 34.5 ± 1.1 pg/mg fw 60 min after the commencement of blue light illumination. During the time course of the experiment, the IAA concentration here also decreased, reaching a basal auxin level after 120 min (24.1 ± 2 pg/mg fw).
To follow the IAA redistribution in response to photostimulation, we measured IAA concentrations in light-exposed and shaded halves of coleoptiles illuminated with blue light under normal gravity and on the rotating BLC (Fig. 2 b and c). In photostimulated plants, the levels of free IAA increased in both halves within the first 5 min after onset of illumination and then returned to the initial level after 15 min (Fig. 2b). Sixty minutes after stimulus onset, auxin redistribution could be resolved. After 120 min, a 1.4 times higher IAA concentration in the shaded than in the light-exposed half was measured. Please note that the IAA concentration in the fast-growing shaded half remained constant, whereas it decreased in the growth-restricted light-exposed side. In seedlings illuminated on the BLC (Fig. 2c), the initial rise in IAA concentration was again less pronounced (compare Fig. 2a). Auxin redistribution was measured after 60 min, too, but in contrast to the plants stimulated under normal gravity, this auxin gradient was the consequence of a significant increase of free IAA in the shaded half and constant levels in the light-exposed half. In the following, the IAA content decreased in both coleoptile halves, but the established gradient was maintained, resulting in a 1.4 times higher IAA concentration in the shaded than in the light-exposed half after 120 min. Here the shaded coleoptile half on the BLC is characterized by an IAA peak at 60 min, whereas the decrease in IAA concentration in the illuminated side is delayed by 30 min when compared with normal gravity.
To explore where the massive rise in IAA after 60 min of blue light illumination on the BLC originates from, we measured IAA concentrations in the light-exposed and shaded halves of coleoptile tips (0–0.5 cm) illuminated with blue light under gravity and on the rotating BLC for 90 min (Fig. 2 d and e). In line with the findings by Philippar et al. (8), the auxin concentration was maximal in the coleoptile tip (50 ± 5 pg/mg fw) and decreased toward the mesocotyl (20 ± 2 pg/mg fw). In photostimulated coleoptile tips, the IAA concentration again increased within 5 min after the onset of blue light illumination and then dropped to the initial level (Fig. 2d). After 60 min, auxin redistribution was monitored. In the tip of seedlings photostimulated on the rotating BLC (Fig. 2e), the initial rise in auxin concentration was not as pronounced as in plants photostimulated under normal gravity. In contrast, the level of free IAA increased slightly on both sides of the tip within the first 30 min after the light had been switched on. Afterward, the level of free IAA increased in the shaded side of the tip, reaching a maximum of 85.7 ± 3.6 pg/mg fw after 60 min, whereas it remained constant in the light-exposed half of the coleoptile tip. Thus, the massive IAA redistribution between the light-exposed and shaded halves of coleoptiles photostimulated on the BLC after 60 min (see Fig. 2c) is mainly a result of IAA relocation within the coleoptile tip.
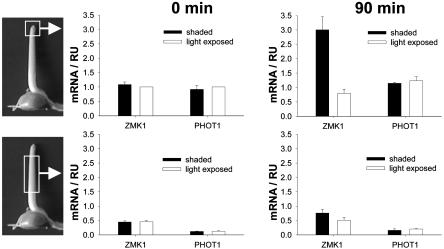
ZMK1 Expression Follows the Auxin Gradient in the Coleoptile Tip. To analyze whether the gradient of ZMK1 expression is also most pronounced in the tip, we monitored ZMK1 transcripts in light-exposed and shaded halves of the photostimulated coleoptile tip (0–0.5 cm) and base (0.5–2 cm) (Fig. 3). Because the blue light signal is perceived by the photoreceptor protein phot1, which has already been shown to be exclusively located in the coleoptile tip (20), we measured PHOT1 expression in the respective samples, too. In control plants (Fig. 3 Left), both ZMK1 and PHOT1 were predominantly expressed in the tip. The content of ZMK1 transcripts in the coleoptile base was only 45% of that found in the tip, and PHOT1 expression was only 15% in the coleoptile base compared with the tip. Transcripts of both genes were evenly distributed in the two flanks of the organ before blue light illumination. Ninety minutes after the onset of irradiation with blue light (Fig. 3 Right), PHOT1 expression was not changed compared with the control plants. In contrast, ZMK1 expression was increased 3-fold in the shaded half of the tip, whereas only a slight differential expression of ZMK1 could be monitored in the coleoptile base. In maize seedlings illuminated with unilateral blue light on the rotating BLC, we found the same expression pattern for ZMK1 and PHOT1 (data not shown). Again, ZMK1 expression was significantly increased in the shaded side of the coleoptile tip after 90 min of irradiation, whereas the distribution of PHOT1 transcripts was not affected by stimulation with blue light.
Fig. 3.
Localization of ZMK1 and PHOT1 expression in the photostimulated coleoptile. Relative transcript levels (RU, relative units) of ZMK1 and PHOT1 in light-exposed (open bars) and shaded (solid bars) halves of the coleoptile tip (Upper) and base (Lower) after 0 (Left) and 90 (Right) min of stimulation with unilateral blue light. The mRNA content (n = 3 ± SE) was quantified as described in Materials and Methods. The level of ZMK1 and PHOT1 mRNA in the light-exposed half at t = 0 min was set to 1.0.
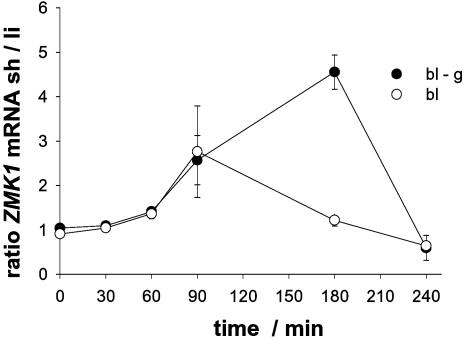
Gravity Alters the Kinetics of Differential ZMK1 Expression. Although gravistimulation and photostimulation on the clinostat resulted in similar bending angles after 4 hr (compare Fig. 1b), ZMK1 ratios were less pronounced in blue light-stimulated coleoptiles after 90 min (see Fig. 1 a and c), when compared with the ZMK1 gradient in gravistimulated plants (8). Therefore, we monitored the time dependence of differential ZMK1 expression during phototropic stimulation under normal and nominally “zero” gravity for up to 4 hr (Fig. 4). After 180 min of blue light stimulation, the ratio of ZMK1 mRNA between the growing and growth-restricted halves of the coleoptile was much more pronounced in plants illuminated on the BLC (4.6 ± 0.4), whereas a ZMK1 mRNA gradient was no longer visible in the coleoptiles photostimulated under gravity. We thus conclude that for coleoptiles incubated on the BLC, prolonged and increased differential expression of ZMK1 seems to account for the stronger bending of the organ (51.5 ± 2.7°), whereas curvature of ≈23° in response to phototropic stimulation under gravity is achieved by a ZMK1 gradient maximal after 90 min of blue light illumination.
Fig. 4.
Time-dependent differential expression of ZMK1 in photostimulated coleoptiles under different gravity conditions. Ratio of ZMK1 transcript level between shaded (sh) and light-exposed (li) sides of phototropically stimulated maize coleoptiles under control conditions (bl, open circles) and on a rotating BLC (bl-g, filled circles) after 0, 30, 60, 90, 180, and 240 min of the corresponding treatment. The mRNA content (n = 3 ± SE) was quantified as described in Materials and Methods.
Discussion
Coleoptile bending in response to a tropistic stimulus results from the differential uptake of potassium in the two flanks of this sensory organ (38). In gravistimulated maize coleoptiles, growth is enhanced in the lower side of the organ (39), where cell elongation is promoted by elevated auxin levels (5, 8), triggering a signal cascade that involves cell wall loosening and the gain of osmolytes. In this context, Claussen et al. (32) have demonstrated that auxin-induced growth of maize coleoptiles depends on the external supply of potassium ions. Following these results, Philippar et al. (8) showed that K+ uptake in the maize coleoptile is mediated by ZMK1, an auxin-induced K+-channel gene, differentially expressed during gravitropic bending of maize coleoptiles. Although the initial rise in cell elongation growth after IAA stimulus is mediated by acidification of the apoplast and thus cell wall loosening as well as the activation of K+-channel proteins, the transcriptional regulation of ZMK1 is thought to play an important role during sustained elongation growth of the coleoptile (for a review, see ref. 14). Focusing on phototropism in this study, we demonstrated that ZMK1 expression also follows the blue light-induced auxin redistribution in the coleoptile tip (Figs. 2 d and e and 3).
ZMK1 mRNA and Auxin Gradients Along the Coleoptile. In accordance with Iino (7), we detected a lateral auxin gradient in the 5-mm apical part of the seedlings in response to stimulation with unilateral blue light, both in plants illuminated under normal gravity and on a rotating clinostat. This auxin redistribution could be correlated with the differential expression of ZMK1 in the illuminated and shaded half of the coleoptile tip (Fig. 3). ZMK1 is predominantly localized in the coleoptile tip, and only here we could detect an induction of ZMK1 expression in the shaded side after 90 min of photostimulation, whereas we found the blue light receptor PHOT1 to be exclusively expressed in the tip but not redistributed on blue light illumination. Although we could detect auxin relocation also in the coleoptile base (data not shown), ZMK1 expression there was only slightly affected. This might be because the auxin concentration in this part of the coleoptile was comparatively low (11.2–26.3 pg/mg fw), below the threshold for the auxin induction of ZMK1 (compare figure 3c of ref. 8).
Time Dependence of Events. In response to the blue light stimulation triggering time-dependent phototropism of maize coleoptiles, a ZMK1 gradient followed the auxin redistribution, which started after 30 min. However, an initial auxin gradient preceding the curvature of maize coleoptiles, found in pulse-induced phototropism, is visible as early as 10 min after stimulus onset (ref. 5; for a review of photoresponses, see ref. 40). The kinetics of differential ZMK1 expression in the process of time-dependent phototropism thus point to a role of this K+ channel during prolonged curvature only. However, signal transduction kinetics leading to ZMK1 expression during phototropism showed distinct differences when compared with the gravity response. A significant gradient of ZMK1 transcripts across the coleoptile was detected 60 min after onset of gravistimulation (compare ref. 8), whereas differential expression of ZMK1 was maximal 90 min after onset of blue light stimulation (Fig. 1 a and c).
ZMK1 Expression Gradient and Magnitude of the Tropistic Response. Differential ZMK1 transcription reached a maximum after 90 min of blue light stimulation under gravity, whereas ZMK1 expression remained asymmetric for at least 180 min in photo-stimulated coleoptiles on a clinostat (Fig. 4). Thus, under blue light stimulation, gravity partially represses differential expression of ZMK1. We therefore conclude that the stronger phototropic bending under nominally “zero” gravity (Fig. 1b) might be due to prolonged differential expression of ZMK1. The ZMK1 mRNA gradient after 180-min blue light stimulation on the BLC (i.e., 4.6:1) was comparable to that after 90-min gravistimulation (6.4:1, see ref. 8), resulting in the same bending angles 4 hr after the onset of the respective stimuli (Fig. 1b). This differential expression of ZMK1 might be due to the massive redistribution of IAA detectable in the shaded coleoptile half after 60 min of blue light illumination on the BLC (compare Fig. 2 c and e). The perception of both stimuli thus results in redistribution of endogenous auxin within the coleoptile tip and finally in asymmetric expression of ZMK1.
The localization of PHOT1 transcripts, auxin redistribution in the coleoptile tip, as well as localization and differential expression of ZMK1 are tightly correlated, leading us to propose the following phototropism model (see Fig. 6, which is published as supporting information on the PNAS web site): K+ and IAA are stored in the maize kernel, IAA mainly in the form of IAA esters like indole-3-acetyl-myo-inositol (IAA-myo-inositol) and IAA-myo-inositol glycosides (41). K+ and IAA-myo-inositol are transported from the kernel into the shoot via the vascular system during coleoptile development (42, 43). IAA is released from IAA-myo-inositol within the coleoptile tip and transported to the responding tissues via polar localized auxin carriers of the AUX- and PIN-families (26, 27). The direction of unilateral illumination is perceived by the blue light receptor phot1, which is evenly distributed within the coleoptile tip. By some unknown signal transduction, this positional signal leads to the relocation of IAA in the coleoptile tip. The signal most likely also activates the enzymes that hydrolyze IAA conjugates, as shown by the increase in IAA concentration within the coleoptile tip 5 min after onset of the gravitropic (8) or phototropic stimulus (Fig. 2 d and e). In turn, the H+-ATPase at the plasma membrane is activated (12). Acidification of the apoplast leads to cell wall loosening, to the activation of the K+-uptake channel ZMK1, and to the initial growth response. During sustained growth, however, the expression of ZMK1 is induced in the shaded half of the organ, resulting in the synthesis of new K+-channel proteins and enhanced K+ uptake of the cells in the shaded coleoptile half, which is a prerequisite for sustained differential cell elongation and in turn the bending of the organ. In search of putative ZMK1 loss-of-function mutants, we have isolated and have just started to analyze a “defective kernel” phenotype, pointing to a central role of ZMK1 in kernel development and growth (K. Philippar, unpublished data).
To conclude, our studies have provided evidence that the spatial and temporal distribution of ZMK1 transcripts and thus differential K+ uptake with respect to both flanks of the coleoptile is able to trigger sustained growth during stimulus-induced bending of this sensory organ.
Supplementary Material
Acknowledgments
This work is dedicated to Winslow Briggs on the occasion of his 75th birthday. We are grateful to R. Granbom for skillful technical assistance and to W. Kaiser and the Julius-von-Sachs-Institute's workshop for constructing the BLC. We also thank W. Briggs and P. Nick for critical discussion and helpful comments on the manuscript. This work was supported by grants from the Deutsche Forschungsgemeinschaft (SPP1067) (to R.H.).
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: BLC, Blue Light Cycler; fw, fresh weight; IAA, indole-3-acetic acid; PHOT1, phototropin 1; ZMK1, Zea mays K+ channel 1.
Data deposition: The sequences reported in this paper have been deposited in the GenBank database [accession nos. Y07632 (ZMK1); AF033263 (PHOT1); AAB40106 (ZmACT81); and AAB40105 (ZmACT83)].
References
- 1.Darwin, C. (1880) The Power of Movements in Plants (Murray, London).
- 2.Went, F. W. (1928) Receuil des Travaux Botaniques Neerlandais 25, 1–116. [Google Scholar]
- 3.Cholodny, N. (1928) Planta 6, 118–134. [Google Scholar]
- 4.Parker, K. E. & Briggs, W. R. (1990) Plant Physiol. 94, 1763–1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iino, M. (1991) Plant Cell Environ. 14, 279–286. [Google Scholar]
- 6.Went, F. W. & Thimann, K. V. (1937) Phytohormones (Macmillan, New York).
- 7.Iino, M. (1995) Plant Cell Physiol. 36, 361–367. [Google Scholar]
- 8.Philippar, K., Fuchs, I., Lüthen, H., Hoth, S., Bauer, C., Haga, K., Thiel, G., Ljung, K., Sandberg, G., Böttger, M., et al. (1999) Proc. Natl. Acad. Sci. USA 96, 12186–12191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mulkey, T. J., Kuzmanoff, K. M. & Evans, M. L. (1981) Planta 152, 239–241. [DOI] [PubMed] [Google Scholar]
- 10.Hager, A., Menzel, H. & Krauss, A. (1971) Planta 100, 47–75. [DOI] [PubMed] [Google Scholar]
- 11.Lohse, G. & Hedrich, R. (1992) Planta 188, 206–214. [DOI] [PubMed] [Google Scholar]
- 12.Felle, H., Peters, W. & Palme, K. (1991) Biochim. Biophys. Acta 1064, 199–204. [DOI] [PubMed] [Google Scholar]
- 13.Frias, I., Caldeira, M. T., Perez-Castineira, J. R., Navarro-Avino, J. P., Culianez-Macia, F. A., Kuppinger, O., Stransky, H., Pages, M., Hager, A. & Serrano, R. (1996) Plant Cell 8, 1533–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Becker, D. & Hedrich, R. (2002) Plant Mol. Biol. 49, 349–356. [PubMed] [Google Scholar]
- 15.Cosgrove, D. J. (2000) Plant Physiol. Biochem. 38, 109–124. [DOI] [PubMed] [Google Scholar]
- 16.Briggs, W. R. & Christie, J. M. (2002) Trends Plant Sci. 7, 204–210. [DOI] [PubMed] [Google Scholar]
- 17.Baum, G., Long, J. C., Jenkins, G. I. & Trewavas, A. I. (1999) Proc. Natl. Acad. Sci. USA 96, 13554–13559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stoelzle, S., Kagawa, T., Wada, M., Hedrich, R. & Dietrich, P. (2003) Proc. Natl. Acad. Sci. USA 100, 1456–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Briggs, W. R., Beck, C. F., Cashmore, A. R., Christie, J. M., Hughes, J., Jarillo, J. A., Kagawa, T., Kanegae, H., Liscum, E., Nagatani A., et al. (2001) Plant Cell 13, 993–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hager, A. & Brich, M. (1993) Planta 189, 567–576. [Google Scholar]
- 21.Kaldenhoff, R. & Iino, M. (1997) Plant Physiol. 114, 1267–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blancaflor, E. B., Fasano, J. M. & Gilroy, S. (1999) Adv. Space Res. 24, 731–738. [DOI] [PubMed] [Google Scholar]
- 23.Marchant, A., Kargul, J., May, S. T., Muller, P., Delbarre, A., Perrot-Rechenmann, C. & Bennett, M. J. (1999) EMBO J. 18, 2066–2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gälweiler, L., Guan, C., Müller, A., Wisman, E., Mendgen, K., Yephremov, A. & Palme, K. (1998) Science 282, 2226–2230. [DOI] [PubMed] [Google Scholar]
- 25.Müller, A., Guan, C., Gälweiler, L., Tanzler, P., Huijser, P., Marchant, A., Parry, G., Bennett, M., Wisman, E. & Palme, K. (1998) EMBO J. 17, 6903–6911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Swarup, R., Friml, J., Marchant, A., Ljung, K., Sandberg, G., Palme, K. & Bennett, M. (2001) Genes Dev. 15, 2648–2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Friml, J., Wisniewska, J., Benkova, E., Mendgen, K. & Palme, K. (2002) Nature 415, 806–809. [DOI] [PubMed] [Google Scholar]
- 28.Friml, J. (2003) Curr. Opin. Plant Biol. 6, 7–12. [DOI] [PubMed] [Google Scholar]
- 29.Woo, E. J., Marshall, J., Bauly, J., Chen, J. G., Venis, M., Napier, R. M. & Pickersgill, R. W. (2002) EMBO J. 21, 2877–2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jones, A. M., Im, K. H., Savka, M. A., Wu, M. J., DeWitt, N. G., Shillito, R. & Binns, A. N. (1998) Science 282, 1114–1117. [DOI] [PubMed] [Google Scholar]
- 31.Chen, J. G., Ullah, H., Young, J. C., Sussman, M. R. & Jones, A. M. (2001) Genes Dev. 15, 902–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Claussen, M., Lüthen, H., Blatt, M. & Böttger, M. (1997) Planta 201, 227–234. [Google Scholar]
- 33.Thiel, G. & Weise, R. (1999) Planta 208, 38–45. [Google Scholar]
- 34.Liu, Y. J. & Iino, M. (1996) Plant Cell Environ. 19, 1379–1388. [DOI] [PubMed] [Google Scholar]
- 35.Philippar, K., Büchsenschütz, K., Abshagen, M., Fuchs, I., Geiger, D., Lacombe, B. & Hedrich, R. (2003) J. Biol. Chem. 278, 16973–16981. [DOI] [PubMed] [Google Scholar]
- 36.Edlund, A., Eklöf, S., Sundberg, B., Moritz, T. & Sandberg, G. (1995) Plant Physiol. 108, 1043–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Iino, M. & Briggs, W. R. (1984) Plant Cell Environ. 7, 97–104. [Google Scholar]
- 38.Goswami, K. K. A. & Audus, L. J. (1976) Ann. Bot. 40, 49–64. [Google Scholar]
- 39.Firn, R. D. & Digby, J. (1979) Plant Cell Environ. 2, 149–154. [DOI] [PubMed] [Google Scholar]
- 40.Iino, M. (2001) in Photomovement, eds. Häder, D. P. & Lebert, M. (Elsevier, Amsterdam), pp. 659–811.
- 41.Epstein, E., Cohen, J. D. & Bandurski, R. S. (1980) Plant Physiol. 65, 415–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nowacki, J. & Bandurski, R. S. (1980) Plant Cell Physiol. 65, 422–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chisnell, J. R. & Bandurski, R. S. (1988) Plant Physiol. 86, 79–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.