Abstract
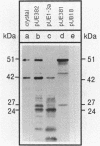
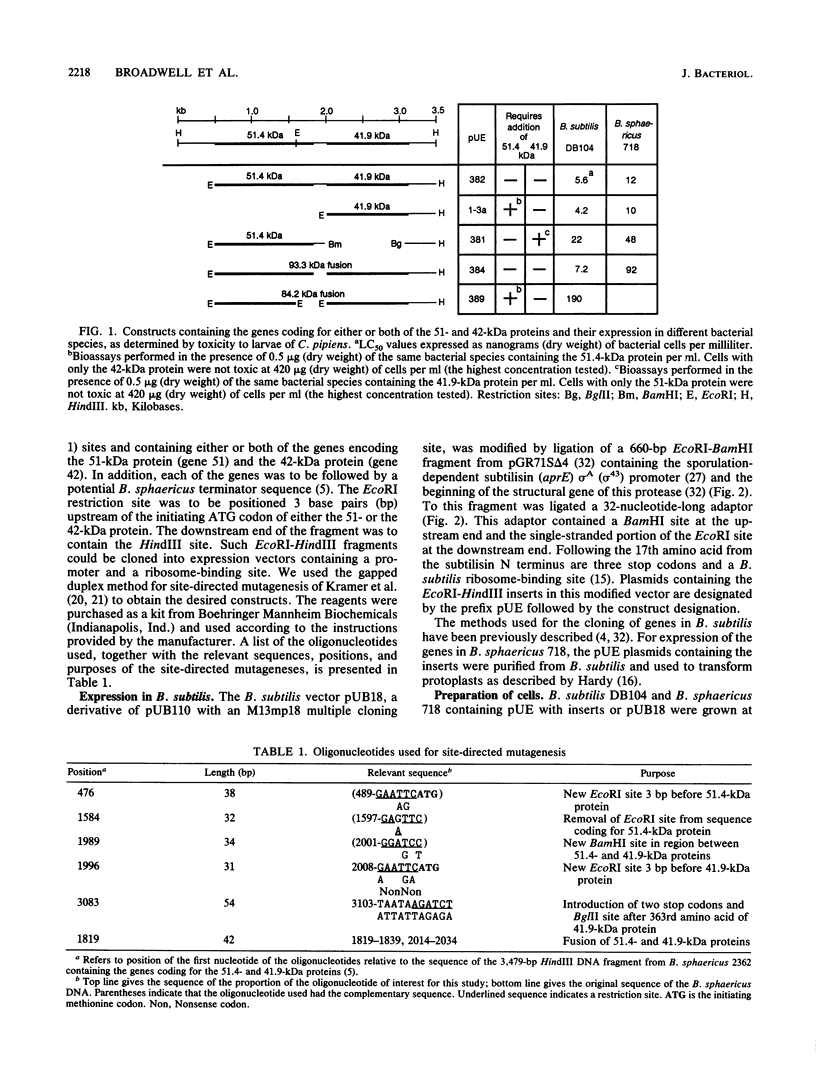
After site-directed mutagenesis, the genes coding for the 42- and 51-kilodalton (kDa) mosquitocidal proteins of Bacillus sphaericus 2362 were placed under the regulation of the aprE (subtilisin) promoter of the Bacillus subtilis vector pUE (a derivative of pUB18). The levels of expression of the gene products in B. subtilis DB104 and B. sphaericus 718 were assessed by bioassays with larvae of Culex pipiens and by Western immunoblots. The results indicated that a higher amount of protein was produced in B. subtilis DB104. Electron microscopic examination of B. subtilis DB104 and B. sphaericus 718 containing the 42- and 51-kDa proteins indicated that amorphous inclusions accumulated in the former species and that crystals identical in appearance to that found in B. sphaericus 2362 were produced in the latter. Strains producing only the 42- or the 51-kDa protein were not toxic to larvae of C. pipiens. A mixture of both strains, a single strain producing both proteins, or a fusion of the 51- and the 42-kDa proteins was toxic. The amount of B. subtilis DB104 containing the 42- and the 51-kDa proteins necessary to kill 50% of the larvae of C. pipiens was 5.6 ng (dry weight) of cells per ml. This value was significantly lower than that for B. sphaericus 2362 (14 ng [dry weight] per ml). Larvae consuming purified amorphous inclusions containing the 42-kDa protein degraded this protein this protein to primarily 39- and 24-kDa peptides, whereas inclusions with the 51-kDa protein were primarily degraded to a protein of 44 kDa. Past studies involving purified proteins from B. sphaericus 2362 indicate an associate of toxicity with the 39-kDa peptide. The results presented here suggest that the 44-kDa degradation product of the 51-kDa protein may also be required for toxicity.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aly C., Mulla M. S., Xu B. Z., Schnetter W. Rate of ingestion by mosquito larvae (Diptera: Culicidae) as a factor in the effectiveness of a bacterial stomach toxin. J Med Entomol. 1988 May;25(3):191–196. doi: 10.1093/jmedent/25.3.191. [DOI] [PubMed] [Google Scholar]
- Amann E., Brosius J., Ptashne M. Vectors bearing a hybrid trp-lac promoter useful for regulated expression of cloned genes in Escherichia coli. Gene. 1983 Nov;25(2-3):167–178. doi: 10.1016/0378-1119(83)90222-6. [DOI] [PubMed] [Google Scholar]
- Arapinis C., de la Torre F., Szulmajster J. Nucleotide and deduced amino acid sequence of the Bacillus sphaericus 1593M gene encoding a 51.4 kD polypeptide which acts synergistically with the 42 kD protein for expression of the larvicidal toxin. Nucleic Acids Res. 1988 Aug 11;16(15):7731–7731. doi: 10.1093/nar/16.15.7731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann L., Baumann P. Expression in Bacillus subtilis of the 51- and 42-kilodalton mosquitocidal toxin genes of Bacillus sphaericus. Appl Environ Microbiol. 1989 Jan;55(1):252–253. doi: 10.1128/aem.55.1.252-253.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann L., Broadwell A. H., Baumann P. Sequence analysis of the mosquitocidal toxin genes encoding 51.4- and 41.9-kilodalton proteins from Bacillus sphaericus 2362 and 2297. J Bacteriol. 1988 May;170(5):2045–2050. doi: 10.1128/jb.170.5.2045-2050.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann P., Baumann L., Bowditch R. D., Broadwell A. H. Cloning of the gene for the larvicidal toxin of Bacillus sphaericus 2362: evidence for a family of related sequences. J Bacteriol. 1987 Sep;169(9):4061–4067. doi: 10.1128/jb.169.9.4061-4067.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann P., Unterman B. M., Baumann L., Broadwell A. H., Abbene S. J., Bowditch R. D. Purification of the larvicidal toxin of Bacillus sphaericus and evidence for high-molecular-weight precursors. J Bacteriol. 1985 Aug;163(2):738–747. doi: 10.1128/jb.163.2.738-747.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowditch R. D., Baumann P., Yousten A. A. Cloning and sequencing of the gene encoding a 125-kilodalton surface-layer protein from Bacillus sphaericus 2362 and of a related cryptic gene. J Bacteriol. 1989 Aug;171(8):4178–4188. doi: 10.1128/jb.171.8.4178-4188.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadwell A. H., Baumann P. Proteolysis in the gut of mosquito larvae results in further activation of the Bacillus sphaericus toxin. Appl Environ Microbiol. 1987 Jun;53(6):1333–1337. doi: 10.1128/aem.53.6.1333-1337.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadwell A. H., Baumann P. Sporulation-associated activation of Bacillus sphaericus larvicide. Appl Environ Microbiol. 1986 Oct;52(4):758–764. doi: 10.1128/aem.52.4.758-764.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles J. F. Ultrastructural midgut events in Culicidae larvae fed with Bacillus sphaericus 2297 spore/crystal complex. Ann Inst Pasteur Microbiol. 1987 Jul-Aug;138(4):471–484. doi: 10.1016/0769-2609(87)90064-0. [DOI] [PubMed] [Google Scholar]
- Davidson E. W. Binding of the Bacillus sphaericus (Eubacteriales: Bacillaceae) toxin to midgut cells of mosquito (Diptera: Culicidae) larvae: relationship to host range. J Med Entomol. 1988 May;25(3):151–157. doi: 10.1093/jmedent/25.3.151. [DOI] [PubMed] [Google Scholar]
- Hindley J., Berry C. Identification, cloning and sequence analysis of the Bacillus sphaericus 1593 41.9 kD larvicidal toxin gene. Mol Microbiol. 1987 Sep;1(2):187–194. doi: 10.1111/j.1365-2958.1987.tb00511.x. [DOI] [PubMed] [Google Scholar]
- Höfte H., Whiteley H. R. Insecticidal crystal proteins of Bacillus thuringiensis. Microbiol Rev. 1989 Jun;53(2):242–255. doi: 10.1128/mr.53.2.242-255.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalfon A., Charles J. F., Bourgouin C., de Barjac H. Sporulation of Bacillus sphaericus 2297: an electron microscope study of crystal-like inclusion biogenesis and toxicity to mosquito larvae. J Gen Microbiol. 1984 Apr;130(4):893–900. doi: 10.1099/00221287-130-4-893. [DOI] [PubMed] [Google Scholar]
- Kramer W., Drutsa V., Jansen H. W., Kramer B., Pflugfelder M., Fritz H. J. The gapped duplex DNA approach to oligonucleotide-directed mutation construction. Nucleic Acids Res. 1984 Dec 21;12(24):9441–9456. doi: 10.1093/nar/12.24.9441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer W., Fritz H. J. Oligonucleotide-directed construction of mutations via gapped duplex DNA. Methods Enzymol. 1987;154:350–367. doi: 10.1016/0076-6879(87)54084-8. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Oeda K., Inouye K., Ibuchi Y., Oshie K., Shimizu M., Nakamura K., Nishioka R., Takada Y., Ohkawa H. Formation of crystals of the insecticidal proteins of Bacillus thuringiensis subsp. aizawai IPL7 in Escherichia coli. J Bacteriol. 1989 Jun;171(6):3568–3571. doi: 10.1128/jb.171.6.3568-3571.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S. S., Wong S. L., Wang L. F., Doi R. H. Bacillus subtilis subtilisin gene (aprE) is expressed from a sigma A (sigma 43) promoter in vitro and in vivo. J Bacteriol. 1989 May;171(5):2657–2665. doi: 10.1128/jb.171.5.2657-2665.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnepf H. E., Whiteley H. R. Delineation of a toxin-encoding segment of a Bacillus thuringiensis crystal protein gene. J Biol Chem. 1985 May 25;260(10):6273–6280. [PubMed] [Google Scholar]
- Tandeau de Marsac N., de la Torre F., Szulmajster J. Expression of the larvicidal gene of Bacillus sphaericus 1593M in the cyanobacterium Anacystis nidulans R2. Mol Gen Genet. 1987 Sep;209(2):396–398. doi: 10.1007/BF00329671. [DOI] [PubMed] [Google Scholar]
- Yousten A. A. Bacillus sphaericus: microbiological factors related to its potential as a mosquito larvicide. Adv Biotechnol Processes. 1984;3:315–343. [PubMed] [Google Scholar]
- Yousten A. A., Davidson E. W. Ultrastructural Analysis of Spores and Parasporal Crystals Formed by Bacillus sphaericus 2297. Appl Environ Microbiol. 1982 Dec;44(6):1449–1455. doi: 10.1128/aem.44.6.1449-1455.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaghloul T. I., Kawamura F., Doi R. H. Translational coupling in Bacillus subtilis of a heterologous Bacillus subtilis-Escherichia coli gene fusion. J Bacteriol. 1985 Nov;164(2):550–555. doi: 10.1128/jb.164.2.550-555.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]