Abstract
In crustaceans, as in most animal species, the amine serotonin has been suggested to serve important roles in aggression. Here we show that injection of serotonin into the hemolymph of subordinate, freely moving animals results in a renewed willingness of these animals to engage the dominants in further agonistic encounters. By multivariate statistical analysis, we demonstrate that this reversal results principally from a reduction in the likelihood of retreat and an increase in the duration of fighting. Serotonin infusion does not alter other aspects of fighting behavior, including which animal initiates an encounter, how quickly fighting escalates, or which animal eventually retreats. Preliminary studies suggest that serotonin uptake plays an important role in this behavioral reversal.
Keywords: aggression, lobsters, crayfish, Prozac
Intraspecific encounters among clawed decapod crustaceans are characterized by a distinct shortage of diplomatic skills. With the exception of mating behavior, most interactions are agonistic in nature, escalating until one of the combatants withdraws. Success is based largely on physical superiority (1–3). Thus, resident populations are bound by a system of dominant/subordinate relationships based on initial agonistic encounters (4, 5). Fights escalate according to rules closely matching predictions of game theory (i.e., sequential assessment strategies), in which animals acquire information about an opponent’s strength and fighting abilities in a stepwise manner (6–10). In this context, the timing of the decision to withdraw by either animal becomes the key element in determining the duration and progress of a fight (6, 8, 9). Decisions may be made after only a brief encounter (seen particularly in the wild) or after prolonged periods of fighting when the physical asymmetries between animals are small. The presence of a highly structured, quantifiable behavioral system in these animals, combined with the potential to bring the analysis to the level of individual neurons (11–16), offers unique vistas in crustaceans for a search for the proximate roots of aggression.
The amine serotonin [5-hydroxytryptamine creatinine sulfate complex (5HT)] has been linked to aggression in a wide and diverse range of species, including humans (17–20). The nature of the linkage, however, is not simple, and it has proven difficult to unravel the role of the amine in the behavior. In vertebrates, lowered levels of 5HT (endogenous or experimentally induced) or changes in amine neuron function that lower the effectiveness of serotonergic neurons generally correlate with increased levels of aggression (19, 20) whereas in invertebrates, the converse is believed to be true (11–13). Genetic alterations of amine neuron function also can change aggressive behavior in animals (21–24) and in people (25–27) although, again, in most cases, it is not clear how the genetic change is linked to the behavior. For example, in humans, a mutation leading to inactivation of one form of the enzyme monoamine oxidase leads to a particular form of explosive violent behavior (26, 27). Because this enzyme is believed to be involved in further metabolism or inactivation of amines, this defect should result in elevated levels of amines, as has been seen in a knockout mutation of the monoamine oxidase enzyme in mice (21). The behavioral manifestation, however, is that generally thought to be associated with lowered levels of 5HT. Finally, direct injections of amines like 5HT into animals also cause changes in aggression, but even here the relationships are complex. For example, in ants, injections of 5HT and its precursors lower interspecific aggressiveness toward intruders but raises intraspecies aggression (28, 29).
Studies examining the role of amines in fighting behavior in crustaceans began with the observation that 5HT and octopamine (OA) injections into freely moving lobsters generated postures resembling those seen when dominant (5HT-like) animals approach subordinates (OA-like) (30, 31). These studies ultimately led to the postulate that amine neuron function might be changed by agonistic interactions between lobsters, with 5HT neuron function becoming more important in dominant animals and OA neuron function more important in subordinates. Recent studies in crayfish demonstrated long term changes in the distribution of 5HT receptor subtypes in specific synaptic regions (14, 15) and changes in excitability of escape reflexes (16) accompanying changes in social status in these animals. With detailed information presently available on the locations of, and physiological roles served by, 5HT and OA neurons in crustaceans (11–13, 32, 33), these systems become even more valuable in the search for linkages between changes in behavior and changes in the functioning of particular neurons and their targets.
Here we report our initial experiments exploring the consequences of amine-specific pharmacological interventions made during agonistic encounters in freely moving lobsters and crayfish. The results show that, for varying periods of time, 5HT injections can reverse subordinate status and induce renewed fighting in decapod crustaceans. Our statistical analyses demonstrate that the behavioral reversal primarily results from altering the decision to retreat from an encounter without changing how fights are initiated, the way in which they escalate, or the final outcome.
MATERIALS AND METHODS
Animals.
Crayfish (Astacus astacus) were obtained from local suppliers in Austria and housed in a holding facility at the Department of Zoology at the University of Graz. Body weights ranged from 12 to 52 g. Recently molted or soon to molt animals (judged by softness of exoskeleton) were not used in any experiments. Experimental animals were isolated in individual pots for at least 5 days before experiments. The pots containing the animals were supplied by partially recirculated water from a central 2000- liter holding tank. Animals were fed twice a week ad libitum with pelleted fish food. Lobsters (Homarus americanus) were grown from hatchlings and were supplied and housed in a rearing facility at The New England Aquarium. These animals are maintained in individual compartments from the 4th stage, when they begin their benthic existence. They are fed daily and are on a 12:12 h day–night cycle, and the animals used in experiments ranged in size from 3 to 30 g.
Observation Chamber.
The observation chambers were either commercial or home-constructed tanks with a clear glass front viewing pane and with continuous running sea water (lobsters) or fresh water (crayfish). The bottoms of the tanks were covered with sand or gravel to a depth of 1 cm. A removable plastic divider separated the tanks into two approximately equal compartments.
Chemicals.
5HT and OA HCl were purchased from commercial sources (Sigma), and Prozac (fluoxetine HCl) was supplied through the generosity of Mrs. M. Niedenthal of the Lilly Research Laboratories, Indianapolis.
Experimental Design and Analysis.
All experiments were recorded on videotape, and, using criteria from previous quantitative analyses (6), individual bouts were analyzed with regard to intensity, duration, which animal initiated an encounter, and which animal retreated. As fights progressed, the intensity was scored according to the following criteria: 0, no fighting: neither animal attacks or one animal retreats quickly from the advances of the other; 1, threat postures: both animals contest the interaction using threat displays or ritualized fighting without using their claws; 2, claw lock: neither animal retreats and at least one animal uses its claws to grab the opponent; 3, strike and rip: both animals contest the encounter and at least one animal makes unrestrained use of claws, attempting to rip or tear off an opponent’s appendages. These data were analyzed as described in the text below.
In the crayfish experiments, 5HT was infused using a syringe pump (Razel Scientific Instruments, Stamford, CT; model A-99) into freely moving animals through indwelling, fused silica, fine bore cannulae. These were implanted into the pericardial sinus of animals 30% smaller than their opponents (average animal sizes were 20 g). In all 19 experiments, the larger animal became dominant during the first 30-min period (preinjection). The subordinate crayfish then were infused with saline for 30 min (control injection at 8 μl/min), followed by saline containing 5HT for 60 min (5HT injection at 8 μl/min with 3 μg of 5HT/min). The infusion pump was turned off after that, and the behavior was monitored for a further 60 min (postinjection). By knowing the rate of infusion and combining that with estimates of blood volume and the t½ of disappearance of 5HT from the hemolymph, we can approximate the hemolymph concentration of 5HT throughout an experiment. About 15 min after the infusion has begun, amine levels stabilize at ≈10−5 M. About 15 min after the pump is switched off, 5HT levels return to baseline.
In the lobster experiments, dominance relationships were established between pairs of animals ranging in size from closely matched to those varying in weight by as much as 30% from each other. We saw no important differences between these groups except that it was more difficult to get clear dominance relationships in the closely matched pairs. After an initial encounter, animals were separated for 30 minutes, and a second encounter was used to confirm the hierarchy. Subordinate animals then were removed from the tanks and injected either with saline (0.45 M NaCl) or with saline containing 5HT at final hemolymph concentrations of 5 × 10−4 to 5 × 10−5 M. After the postural changes produced by 5HT injections decayed away (30–45 min later), subordinate animals were placed back in contact with the previous dominants.
RESULTS AND DISCUSSION
Effects of Amine Infusion on Fighting Behavior.
Two sets of experiments, one with crayfish and the other with lobsters, demonstrated that infusing or injecting 5HT into the hemolymph of subordinate animals restores fighting to levels significantly exceeding those characteristic of established dominance relationships. The focus in this article is on the crayfish studies. A summary of changes in duration and intensity of crayfish encounters over time is shown in Fig. 1. During the preinjection period, dominance was established with little fighting, and interactions rarely lasted longer than a few minutes because of the differences in body size. The infusion of saline did not alter the levels of aggression. In contrast, the continuous infusion of 5HT into a subordinate animal was accompanied by a steady increase in fighting, with the duration and intensity peaking at levels three times the preinjection average (Fig. 1, upper graph). The fight-enhancing effects of 5HT were reversed by 30 min after the infusion was turned off and the levels of aggression returned to baseline, preinjection levels. As anticipated, OA infusion into subordinate animals did not enhance their fighting behavior (Fig. 1, lower graph). The effects of OA injection into dominant animals has not yet been determined. In the lobster experiments, which are not illustrated here, the results we observed were qualitatively similar to those seen in the crayfish fights; significant increases were found in both the duration and intensity of fights after 5HT injection.
Figure 1.
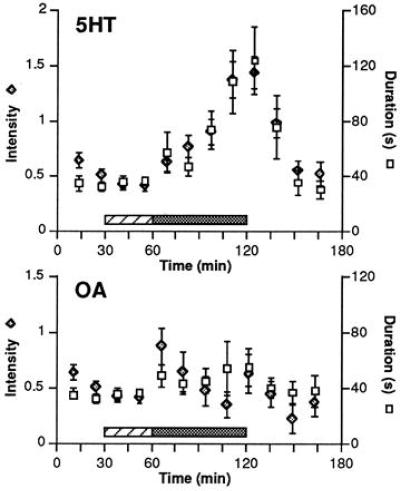
Serotonin effects on crayfish fighting: Univariate analyses. Mean ± SE of intensity (maximum level reached during each interaction) and duration from 1453 agonistic interactions are summarized for infusions of 5HT (Upper) and OA (Lower). Dominance is established within the first 30 min of the experiment (preinjection period) and escalated fighting subsides. No increases in fighting behavior are observed during saline infusion (0.125 M NaCl, hatched period). The infusion of 5HT in saline into the subordinate animal (Upper, gray area) is accompanied by a constant rise in the duration and intensity of agonistic encounters. After the 5HT infusion is turned off, the levels of fighting return to those of the preinjection period within 30 minutes. Infusion of OA into subordinate animals (Lower, gray area) does not significantly alter fighting.
Multivariate Analysis.
From our previous results (6), we know that fights escalate and reach increasing levels of intensity the longer they last (i.e., duration and intensity are closely correlated). When each variable is examined independently (univariate analysis), we observed increases in both the duration and intensity of fighting with 5HT infusion, as shown in Fig. 1. The univariate analysis cannot tell us, however, whether the relationship between the two variables is altered by amine injection or whether the variables are equally important in the interpretation of the amine effect. Therefore, with multivariate statistical techniques, we (i) characterized the relationships between the behavioral variables using principal components analysis and (ii) identified the behavior that was changed most by the experimental manipulations using discriminant function analysis. The specific details of these analyses will be shown in a later publication. Here, the results are graphed as a canonical centroid plot (Fig. 2) that demonstrates that an increase in fight duration was the most important variable influenced by the amine. Variation in intensity or in which animal initiated or which retreated from fights contributed little additional information to that already contained in duration. Thus 5HT selectively decreased the likelihood that subordinates withdraw from the attacks of their dominant opponents without altering their locomotor activity, the rules of escalation of fights, or the eventual outcome of an encounter.
Figure 2.
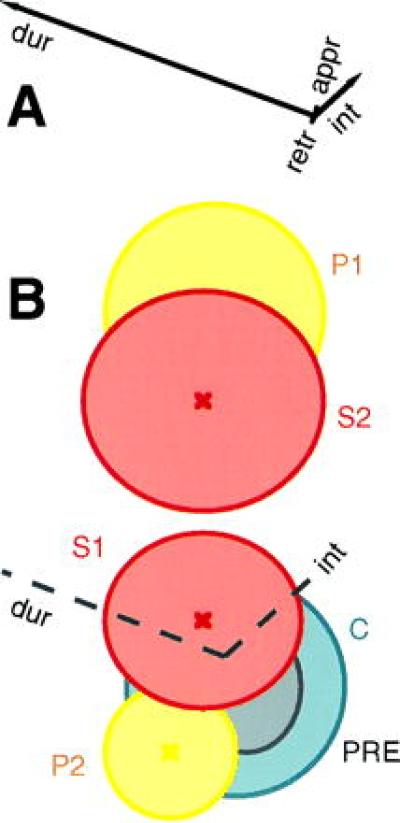
Serotonin effects on crayfish fighting: Multivariate analysis. When 5HT is infused into the hemolymph of crayfish, all behavioral characteristics of fighting change significantly. Measures of intensity and duration are highly correlated in escalated fights and are dependent on the identity of the animal initiating the encounter or retreating. Therefore, a multivariate approach was used to identify the behavioral aspects most altered as a result of 5HT infusion. For this analysis, interactions were assigned to one of six experimental groups: preinjection (PRE, gray), control injection (C, blue), first (S1, red) or second (S2, red) half hour of 5HT infusion, and first (P1, yellow) or second (P2, yellow) half hour of post injection. Discriminant function analysis identified the behavioral characteristics that were most useful in distinguishing among the treatment groups. The results are summarized as a canonical centroid plot, in which each measured variable (e.g., duration or intensity of interactions) is indicated as a vector whose length represents its relative importance. (A) Significant separation is provided by the duration (dur) of the interactions with little additional information contributed by the intensity (int), which animal approaches (appr) or which retreats (retr). (B) The agonistic interactions for each treatment group are summarized as a multivariate mean (centroid) with 95% confidence limits. The duration vector is not shown at its full length in this graph. The overlap of the preinjection (PRE), control injection (C), and second postinjection (P2) periods indicates a similarity in their behavioral characteristics. With 5HT infusion, the fights move from the center of the graph toward longer durations, with only minor contributions from changes in intensity.
Mechanism of the Behavioral Reversal: Preliminary Experiments with Prozac.
The timing of the decision to withdraw from an encounter is a key element of game theory models of fighting (7–10). Our results raise the possibility that decision making of this sort may involve a balance between 5HT and other neuromodulatory substances (e.g., OA?) at key sites in the nervous systems of these animals. Our presumption is that we disturb that balance with injections of 5HT. We do not believe that residual free amine circulating in the hemolymph causes the behavioral reversal. The t½ of clearance of 5HT from the circulation (lobster ≈6 minutes; crayfish ≈11 minutes) argues against that possibility. Other mechanisms that could be involved are: (i) delayed second messenger-mediated effects in key decision-making neurons; (ii) an uptake of 5HT into serotonergic nerve terminals elevating amine levels, with subsequent release of higher levels of the amine during later agonistic encounters (22); and (iii) physiological actions of the sulfated metabolites of 5HT, which are released into the hemolymph after their formation in many crustacean tissues (34).
We have carried out preliminary experiments exploring possibility (ii) using Prozac (35). As in vertebrate systems, Prozac blocks 5HT uptake into serotonergic nerve terminals in lobsters (34). We have examined the effects of acute injections or infusions of Prozac alone and Prozac in combination with 5HT into subordinate animals (Fig. 3). Prozac alone has no effect on fighting behavior. This is not surprising because acute Prozac has little effect on patients either (35). In contrast, the effects of 5HT injections are significantly reduced in the presence of Prozac, suggesting that uptake plays an important role in the behavioral reversals (Fig. 3).
Figure 3.
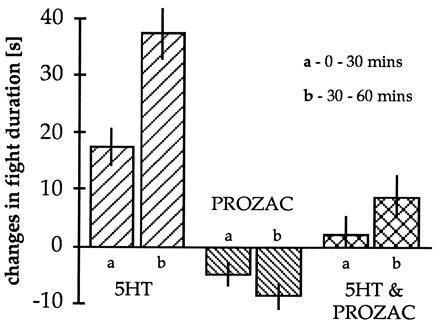
Changes in mean fight duration with 5HT and Prozac treatment. Each pair of bars represents the mean fight duration during the first and second 30 min of treatment with the agent. Compared with the average duration of fights in control (preinfusion) periods (average, 41 s), the length of encounters increases in the presence of 5HT (left two bars). Acute Prozac (final hemolymph concentration 1–2 × 10−5 M) results in a slight decrease in fight duration (middle two bars). The infusion of Prozac together with 5HT led to a significant reduction in the 5HT-mediated increases in fight duration (right two bars). As anticipated, similar results were seen in measurements of fight intensity (not shown).
Aggressive Motivation and Decision-Making Centers.
Under normal circumstances, animals faced with large asymmetries in body size, vigor, stamina, or fighting dexterity quickly retreat from advancing opponents. Failure to withdraw from an encounter when faced with a superior foe brings about substantial increases in the risk of injury. 5HT-injected crayfish and lobsters continued to engage their opponents and continued fighting in situations that ordinarily would result in withdrawal. It appears, therefore, that, for a period of time, amine injection altered the animals’ decision to retreat and behave as a subordinate. Thus, with 5HT injection into subordinate animals, we believe that we have changed the animals’ “willingness to fight” or “aggressive motivation.”
Important questions remain concerning the particular amine neurons responsible for such changes in decision making. The relevant serotonergic neurons probably are not those located in the abdominal or thoracic ganglia that have been the focus of our earlier studies (11–13). These 5HT neurons seem mainly concerned with postural regulation. Instead, of particular interest may be a pair of deutocerebral giant serotonergic neurons found in the supraesophageal ganglion (brain) of crayfish and lobsters. Each of these neurons densely innervate two central nervous system neuropil regions, the olfactory and accessory lobes (36). The latter, a synaptic center that receives no primary sensory input (36), has been postulated to serve in higher function decision making in these species. Further detailed explorations of deutocerebral giants and their target synaptic regions will be required, however, before we will know whether higher order decisions, like when to give up in an agonistic encounter, lie in these central processing centers.
In crustacean species, animals that win multiple encounters are more likely to win subsequent ones (2, 4). They show an increased willingness to engage in agonistic encounters and in that way resemble our 5HT-injected subordinate animals. Our present results, when coupled with our earlier studies on the role of amines in postural regulation (11–13) and those of other investigators showing changes of 5HT receptor distribution and of giant axon-mediated escape responsiveness accompanying fight-induced changes in the status of animals (14–16), offer strong support for an important role of 5HT in fighting behavior of decapod crustaceans. The summed results demonstrate further that, through the use of model systems of this sort, the roots of complex behaviors like aggression and answers to questions relating to the neurobiological basis of motivation become approachable at levels difficult to achieve in higher forms.
Acknowledgments
We thank Jason Goldstein and Marianne Farrington from the New England Aquarium for their assistance in providing animals. We also thank Magdalena Orzeszyna for carrying out preliminary behavioral experiments, Dr. Rami Rahamimoff for suggestions on data analysis and interpretation, and Dr. Moira J. van Staaden for review of the manuscript. The lobster experiments were performed at a rearing facility in the New England Aquarium. K.I. was supported by a travel grant from the Karolinska Institute. K.S. was supported by a Four Directions Summer Program award from Harvard Medical School. Other support was from Austrian Science Foundation 10165-BIO and Jubilaeumsfonds of the Austrian National Bank 5303 (both to R.H.) and from National Institutes of Health Grant NS-25915 and National Science Foundation Grant IBN-9601288 (both to E.A.K.). All animal experiments were performed under guidelines approved by Harvard Medical School and the New England Aquarium.
ABBREVIATIONS
- 5HT
serotonin
- OA
octopamine
References
- 1.Bovbjerg R V. Physiol Zool. 1956;29:127–136. [Google Scholar]
- 2.Scrivener J C E. Fish Res Board Can Tech Rep. 1971;235:1–128. [Google Scholar]
- 3.Evans D L, Shehadi-Moacdieh M. Anim Behav. 1988;36:452–455. [Google Scholar]
- 4.Atema J, Cobb J S. In: The Biology and Management of Lobsters. Cobb J S, Phillips B F, editors. New York: Academic; 1980. pp. 409–450. [Google Scholar]
- 5.Figler M H, Finkelstein J E, Twum M, Peeke H V S. Aggr Behav. 1995;21:225–236. [Google Scholar]
- 6.Huber R, Kravitz E A. Brain Behav Evol. 1995;46:72–83. doi: 10.1159/000113260. [DOI] [PubMed] [Google Scholar]
- 7.Maynard-Smith J. Evolution and the Theory of Games. Cambridge, UK: Cambridge Univ. Press; 1982. [Google Scholar]
- 8.Parker G A. J Theor Biol. 1974;47:223–243. doi: 10.1016/0022-5193(74)90111-8. [DOI] [PubMed] [Google Scholar]
- 9.Parker G A, Rubinstein D I. Anim Behav. 1981;29:221–240. [Google Scholar]
- 10.Austad S N. Trends Ecol Evol. 1989;4:2–3. [Google Scholar]
- 11.Kravitz E A. Science. 1988;241:1775–1781. doi: 10.1126/science.2902685. [DOI] [PubMed] [Google Scholar]
- 12.Beltz B S, Kravitz E A. J Neurosci. 1983;3:585–602. doi: 10.1523/JNEUROSCI.03-03-00585.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ma P M, Beltz B S, Kravitz E A. J Neurophys. 1992;68:36–54. doi: 10.1152/jn.1992.68.1.36. [DOI] [PubMed] [Google Scholar]
- 14.Yeh S, Fricke R A, Edwards D H. Science. 1996;271:366–369. doi: 10.1126/science.271.5247.366. [DOI] [PubMed] [Google Scholar]
- 15.Yeh S, Musolf B E, Edwards D H. J Neurosci. 1997;17:697–708. doi: 10.1523/JNEUROSCI.17-02-00697.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krasne F B, Shamsian A, Kulkarni R. J Neurosci. 1997;17:709–716. doi: 10.1523/JNEUROSCI.17-02-00709.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coccaro E F. Br J Psychiatry. 1989;155:52–62. [PubMed] [Google Scholar]
- 18.Golden, R. N., Gilmore, J. H., Corrigan, M. H., Ekstrom, R. D., Knight, B. T. & Garbutt, J. C. (1991) J. Clin. Psychol. 52, Suppl., 61–69. [PubMed]
- 19.Raleigh M J, Mcguire M T, Brammer G L, Pollack D B, Yuwiler A. Brain Res. 1991;559:181–190. doi: 10.1016/0006-8993(91)90001-c. [DOI] [PubMed] [Google Scholar]
- 20.Miczek K A, Weerts E, Haney M, Tidey J. Neurosci Biobehav Rev. 1994;18:97–110. doi: 10.1016/0149-7634(94)90040-x. [DOI] [PubMed] [Google Scholar]
- 21.Cases O, Seif I, Grimsby J, Gaspar P, Chen K, Pournin S, Muller U, Aguet M, Babinet C, Shih J C, Demaeyer E. Science. 1995;268:1763–1766. doi: 10.1126/science.7792602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen C, Rainnie D G, Greene R W, Tonegawa S. Science. 1994;266:291–294. doi: 10.1126/science.7939668. [DOI] [PubMed] [Google Scholar]
- 23.Saudou F, Amara D A, Dierich A, LeMeur M, Ramboz S, Segu L, Buhot M, Hen R. Science. 1994;265:1875–1878. doi: 10.1126/science.8091214. [DOI] [PubMed] [Google Scholar]
- 24.Nelson R J, Demas G E, Huang P L, Fishman M C, Dawson V L, Dawson T M, Snyder S H. Nature (London) 1995;378:383–386. doi: 10.1038/378383a0. [DOI] [PubMed] [Google Scholar]
- 25.Nielsen D A, Goldman D, Virkkunen M, Tokola R, Rawlings R, Linnoila M. Arch Gen Psychiatry. 1994;51:34–38. doi: 10.1001/archpsyc.1994.03950010034005. [DOI] [PubMed] [Google Scholar]
- 26.Brunner H G, Nelen M, Breakfield X O, Ropers H H, van Oost B A. Science. 1993;262:578–580. doi: 10.1126/science.8211186. [DOI] [PubMed] [Google Scholar]
- 27.Brunner H G, Nelen M R, van Zandvoort P, Abeling N G, van Gennip A H, Wolters E C, Kuiper M A, Ropers H H, van Oost B A. Am J Hum Genet. 1993;52:1032–1039. [PMC free article] [PubMed] [Google Scholar]
- 28.Kostowski W, Tarchalska B. Brain Res. 1972;38:143–149. doi: 10.1016/0006-8993(72)90595-1. [DOI] [PubMed] [Google Scholar]
- 29.Kostowski W, Tarchalska-Krynska B, Markowska L. Pharmacol Biochem Behav. 1975;3:717–719. doi: 10.1016/0091-3057(75)90200-2. [DOI] [PubMed] [Google Scholar]
- 30.Livingstone M S, Harris-Warrick R M, Kravitz E A. Science. 1980;208:76–79. doi: 10.1126/science.208.4439.76. [DOI] [PubMed] [Google Scholar]
- 31.Harris-Warrick R M, Kravitz E A. J Neurosci. 1984;4:1976–1993. doi: 10.1523/JNEUROSCI.04-08-01976.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Beltz B S, Kravitz E A. J Neurosci. 1987;7:533–546. doi: 10.1523/JNEUROSCI.07-02-00533.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schneider H, Trimmer B A, Rapus J, Eckert M, Valentine D F, Kravitz E A. J Comp Neurol. 1993;329:129–142. doi: 10.1002/cne.903290109. [DOI] [PubMed] [Google Scholar]
- 34.Huber, R., Orzeszyna, M., Pokorny, N. & Kravitz, E. A. (1997) Brain Behav. Evol., in press. [DOI] [PubMed]
- 35.Stokes P E. Clin Ther. 1993;15:216–241. [PubMed] [Google Scholar]
- 36.Sandeman D C, Sandeman R E. J Comp Neurol. 1994;341:130–144. doi: 10.1002/cne.903410111. [DOI] [PubMed] [Google Scholar]


