Abstract
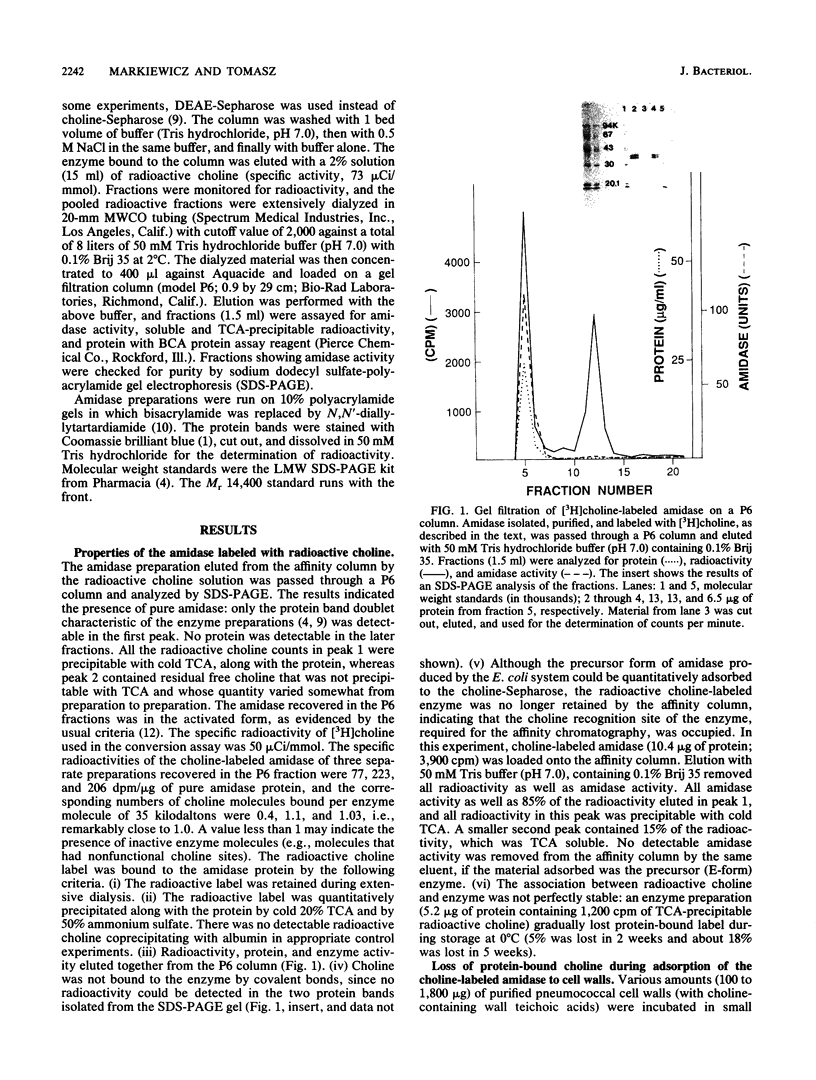
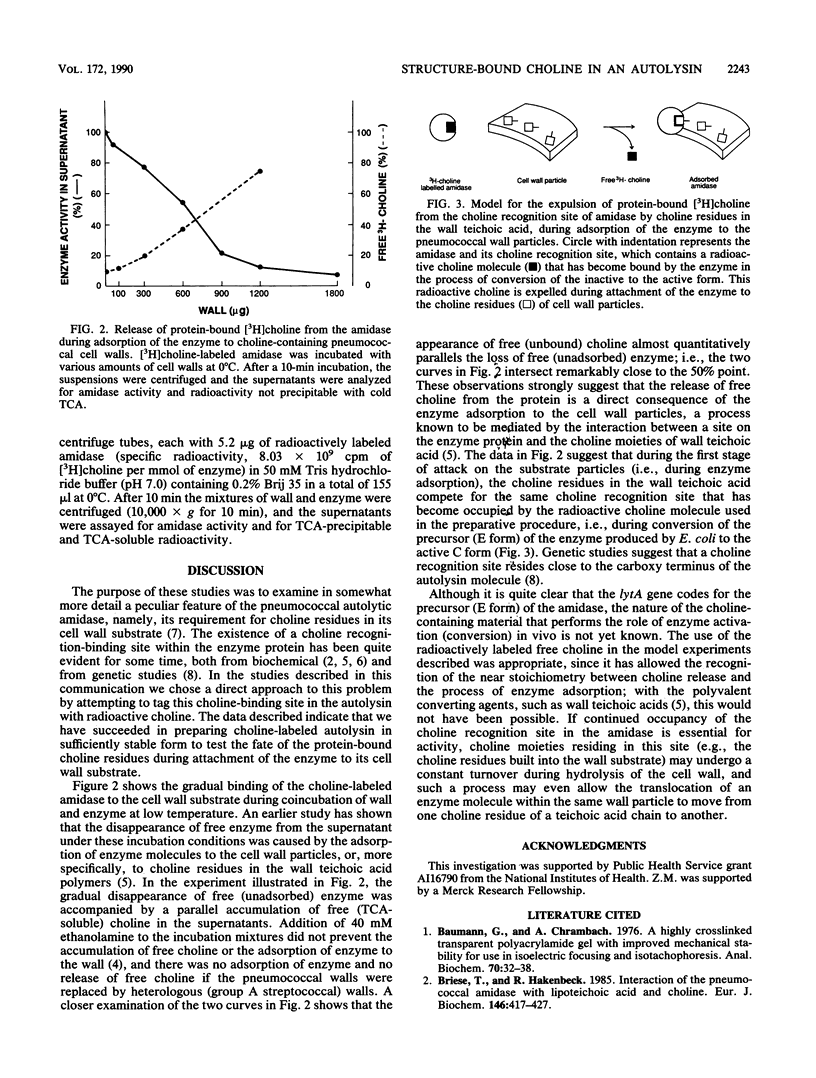
The inactive precursor form of the pneumococcal autolytic enzyme cloned in Escherichia coli was isolated by affinity chromatography on Sepharose-linked choline. The enzyme was recovered in an electrophoretically pure and activated form by elution from the affinity column with radioactive choline solution. When radioactive choline was used for elutions, the enzyme protein isolated contained protein-bound choline, at approximately 1 mol of choline per mol of enzyme protein, indicating the presence of a single choline recognition site. Radioactive choline remained bound to the enzyme protein during dialysis, precipitation by trichloroacetic acid or ammonium sulfate, and during gel filtration, but not during sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Incubation of the choline-labeled autolysin with pneumococcal cell walls at 0 degrees C resulted in the adsorption of the enzyme to the wall particles and a simultaneous release of free choline from the enzyme protein. It is suggested that the choline molecules that became bound to the enzyme protein during the activation of autolysin are expelled from the choline-binding site and replaced by choline residues from the wall teichoic acid as the autolysin molecules adsorb to their insoluble substrate before the onset of enzymatic wall hydrolysis.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baumann G., Chrambach A. A highly crosslinked, transparent polyacrylamide gel with improved mechanical stability for use in isoelectric focusing and isotachophoresis. Anal Biochem. 1976 Jan;70(1):32–38. doi: 10.1016/s0003-2697(76)80044-9. [DOI] [PubMed] [Google Scholar]
- Briese T., Hakenbeck R. Interaction of the pneumococcal amidase with lipoteichoic acid and choline. Eur J Biochem. 1985 Jan 15;146(2):417–427. doi: 10.1111/j.1432-1033.1985.tb08668.x. [DOI] [PubMed] [Google Scholar]
- Brundish D. E., Baddiley J. Pneumococcal C-substance, a ribitol teichoic acid containing choline phosphate. Biochem J. 1968 Dec;110(3):573–582. doi: 10.1042/bj1100573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García J. L., García E., López R. Overproduction and rapid purification of the amidase of Streptococcus pneumoniae. Arch Microbiol. 1987;149(1):52–56. doi: 10.1007/BF00423136. [DOI] [PubMed] [Google Scholar]
- Giudicelli S., Tomasz A. Attachment of pneumococcal autolysin to wall teichoic acids, an essential step in enzymatic wall degradation. J Bacteriol. 1984 Jun;158(3):1188–1190. doi: 10.1128/jb.158.3.1188-1190.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höltje J. V., Tomasz A. Specific recognition of choline residues in the cell wall teichoic acid by the N-acetylmuramyl-L-alanine amidase of Pneumococcus. J Biol Chem. 1975 Aug 10;250(15):6072–6076. [PubMed] [Google Scholar]
- Mosser J. L., Tomasz A. Choline-containing teichoic acid as a structural component of pneumococcal cell wall and its role in sensitivity to lysis by an autolytic enzyme. J Biol Chem. 1970 Jan 25;245(2):287–298. [PubMed] [Google Scholar]
- Sanz J. M., Lopez R., Garcia J. L. Structural requirements of choline derivatives for 'conversion' of pneumococcal amidase. A new single-step procedure for purification of this autolysin. FEBS Lett. 1988 May 23;232(2):308–312. doi: 10.1016/0014-5793(88)80759-2. [DOI] [PubMed] [Google Scholar]
- Späth P. J., Koblet H. Properties of SDS-polyacrylamide gels highly cross-linked with N,N'-diallyltartardiamide and the rapid isolation of macromolecules from the gel matrix. Anal Biochem. 1979 Mar;93(2):275–285. doi: 10.1016/s0003-2697(79)80152-9. [DOI] [PubMed] [Google Scholar]
- Sánchez-Puelles J. M., García J. L., López R., García E. 3'-end modifications of the Streptococcus pneumoniae lytA gene: role of the carboxy terminus of the pneumococcal autolysin in the process of enzymatic activation (conversion). Gene. 1987;61(1):13–19. doi: 10.1016/0378-1119(87)90360-x. [DOI] [PubMed] [Google Scholar]
- Tomasz A. Choline in the cell wall of a bacterium: novel type of polymer-linked choline in Pneumococcus. Science. 1967 Aug 11;157(3789):694–697. doi: 10.1126/science.157.3789.694. [DOI] [PubMed] [Google Scholar]
- Tomasz A., Westphal M. Abnormal autolytic enzyme in a pneumococus with altered teichoic acid composition. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2627–2630. doi: 10.1073/pnas.68.11.2627. [DOI] [PMC free article] [PubMed] [Google Scholar]