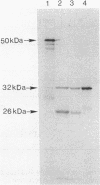
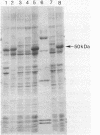
Abstract
A 3.8-kilobase DNA fragment from Bacillus subtilis containing the hemA gene has been cloned and sequenced. Four open reading frames were identified. The first is hemA, encoding a protein of 50.8 kilodaltons. The primary defect of a B. subtilis 5-aminolevulinic acid-requiring mutant was identified as a cysteine-to-tyrosine substitution in the HemA protein. The predicted amino acid sequence of the B. subtilis HemA protein showed 34% identity with the Escherichia coli HemA protein, which is known to code for the NAD(P)H:glutamyl-tRNA reductase of the C5 pathway for 5-aminolevulinic acid synthesis. The B. subtilis HemA protein also complements the defect of an E. coli hemA mutant. The second open reading frame in the cloned fragment, called ORF2, codes for a protein of about 30 kilodaltons with unknown function. It is not the proposed hemB gene product porphobilinogen synthase. The third open reading frame is hemC, coding for porphobilinogen deaminase. The fourth open reading frame extends past the sequenced fragment and may be identical to hemD, coding for uroporphyrinogen III cosynthase. Analysis of deletion mutants of the hemA region suggests that (at least) hemA, ORF2, and hemC may be part of an operon.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson T. J., Ivánovics G. Isolation and some characteristics of haemin dependent mutants of Bacillus subtilis. J Gen Microbiol. 1967 Oct;49(1):31–40. doi: 10.1099/00221287-49-1-31. [DOI] [PubMed] [Google Scholar]
- Arwert F., Venema G. Transformation in Bacillus subtilis. Fate of newly introduced transforming DNA. Mol Gen Genet. 1973;123(2):185–198. doi: 10.1007/BF00267334. [DOI] [PubMed] [Google Scholar]
- Avissar Y. J., Beale S. I. Identification of the enzymatic basis for delta-aminolevulinic acid auxotrophy in a hemA mutant of Escherichia coli. J Bacteriol. 1989 Jun;171(6):2919–2924. doi: 10.1128/jb.171.6.2919-2924.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avissar Y. J., Ormerod J. G., Beale S. I. Distribution of delta-aminolevulinic acid biosynthetic pathways among phototrophic bacterial groups. Arch Microbiol. 1989;151(6):513–519. doi: 10.1007/BF00454867. [DOI] [PubMed] [Google Scholar]
- Bawden M. J., Borthwick I. A., Healy H. M., Morris C. P., May B. K., Elliott W. H. Sequence of human 5-aminolevulinate synthase cDNA. Nucleic Acids Res. 1987 Oct 26;15(20):8563–8563. doi: 10.1093/nar/15.20.8563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beale S. I., Castelfranco P. A. The Biosynthesis of delta-Aminolevulinic Acid in Higher Plants: II. Formation of C-delta-Aminolevulinic Acid from Labeled Precursors in Greening Plant Tissues. Plant Physiol. 1974 Feb;53(2):297–303. doi: 10.1104/pp.53.2.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berek I., Miczák A., Kiss I., Ivánovics G., Durkó I. Genetic and biochemical analysis of haemin dependent mutants of Bacillus subtilis. Acta Microbiol Acad Sci Hung. 1975;22(2):157–167. [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Betlach M. C., Boyer H. W. Construction and characterization of new cloning vehicles. I. Ampicillin-resistant derivatives of the plasmid pMB9. Gene. 1977;2(2):75–93. doi: 10.1016/0378-1119(77)90074-9. [DOI] [PubMed] [Google Scholar]
- Capaldi R. A., Vanderkooi G. The low polarity of many membrane proteins. Proc Natl Acad Sci U S A. 1972 Apr;69(4):930–932. doi: 10.1073/pnas.69.4.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dailey H. A., Karr S. W. Purification and characterization of murine protoporphyrinogen oxidase. Biochemistry. 1987 May 19;26(10):2697–2701. doi: 10.1021/bi00384a007. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drolet M., Péloquin L., Echelard Y., Cousineau L., Sasarman A. Isolation and nucleotide sequence of the hemA gene of Escherichia coli K12. Mol Gen Genet. 1989 Apr;216(2-3):347–352. doi: 10.1007/BF00334375. [DOI] [PubMed] [Google Scholar]
- Echelard Y., Dymetryszyn J., Drolet M., Sasarman A. Nucleotide sequence of the hemB gene of Escherichia coli K12. Mol Gen Genet. 1988 Nov;214(3):503–508. doi: 10.1007/BF00330487. [DOI] [PubMed] [Google Scholar]
- Elliott T. Cloning, genetic characterization, and nucleotide sequence of the hemA-prfA operon of Salmonella typhimurium. J Bacteriol. 1989 Jul;171(7):3948–3960. doi: 10.1128/jb.171.7.3948-3960.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott T., Roth J. R. Heme-deficient mutants of Salmonella typhimurium: two genes required for ALA synthesis. Mol Gen Genet. 1989 Apr;216(2-3):303–314. doi: 10.1007/BF00334369. [DOI] [PubMed] [Google Scholar]
- Ferreira G. C., Andrew T. L., Karr S. W., Dailey H. A. Organization of the terminal two enzymes of the heme biosynthetic pathway. Orientation of protoporphyrinogen oxidase and evidence for a membrane complex. J Biol Chem. 1988 Mar 15;263(8):3835–3839. [PubMed] [Google Scholar]
- Granick S., Beale S. I. Hemes, chlorophylls, and related compounds: biosynthesis and metabolic regulation. Adv Enzymol Relat Areas Mol Biol. 1978;46:33–203. doi: 10.1002/9780470122914.ch2. [DOI] [PubMed] [Google Scholar]
- Grimm B., Bull A., Welinder K. G., Gough S. P., Kannangara C. G. Purification and partial amino acid sequence of the glutamate 1-semialdehyde aminotransferase of barley and synechococcus. Carlsberg Res Commun. 1989;54(2):67–79. doi: 10.1007/BF02907586. [DOI] [PubMed] [Google Scholar]
- Guarente L., Mason T. Heme regulates transcription of the CYC1 gene of S. cerevisiae via an upstream activation site. Cell. 1983 Apr;32(4):1279–1286. doi: 10.1016/0092-8674(83)90309-4. [DOI] [PubMed] [Google Scholar]
- Holmgren E., Hederstedt L., Rutberg L. Role of heme in synthesis and membrane binding of succinic dehydrogenase in Bacillus subtilis. J Bacteriol. 1979 May;138(2):377–382. doi: 10.1128/jb.138.2.377-382.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ish-Horowicz D., Burke J. F. Rapid and efficient cosmid cloning. Nucleic Acids Res. 1981 Jul 10;9(13):2989–2998. doi: 10.1093/nar/9.13.2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan P. M., Mgbeje B. I., Alwan A. F., Thomas S. D. Nucleotide sequence of hemD, the second gene in the hem operon of Escherichia coli K-12. Nucleic Acids Res. 1987 Dec 23;15(24):10583–10583. doi: 10.1093/nar/15.24.10583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannangara C. G., Gough S. P., Bruyant P., Hoober J. K., Kahn A., von Wettstein D. tRNA(Glu) as a cofactor in delta-aminolevulinate biosynthesis: steps that regulate chlorophyll synthesis. Trends Biochem Sci. 1988 Apr;13(4):139–143. doi: 10.1016/0968-0004(88)90071-0. [DOI] [PubMed] [Google Scholar]
- Kieser T. Factors affecting the isolation of CCC DNA from Streptomyces lividans and Escherichia coli. Plasmid. 1984 Jul;12(1):19–36. doi: 10.1016/0147-619x(84)90063-5. [DOI] [PubMed] [Google Scholar]
- Kiss I., Berek I., Ivánovics G. Mapping the -aminolaevulinic acid synthetase locus in Bacillus subtilis. J Gen Microbiol. 1971 May;66(2):153–159. doi: 10.1099/00221287-66-2-153. [DOI] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Li J. M., Russell C. S., Cosloy S. D. Cloning and structure of the hem A gene of Escherichia coli K-12. Gene. 1989 Oct 30;82(2):209–217. doi: 10.1016/0378-1119(89)90046-2. [DOI] [PubMed] [Google Scholar]
- Li J. M., Russell C. S., Cosloy S. D. The structure of the Escherichia coli hemB gene. Gene. 1989 Jan 30;75(1):177–184. doi: 10.1016/0378-1119(89)90394-6. [DOI] [PubMed] [Google Scholar]
- Li J. M., Umanoff H., Proenca R., Russell C. S., Cosloy S. D. Cloning of the Escherichia coli K-12 hemB gene. J Bacteriol. 1988 Feb;170(2):1021–1025. doi: 10.1128/jb.170.2.1021-1025.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacGregor C. H. Biosynthesis of membrane-bound nitrate reductase in Escherichia coli: evidence for a soluble precursor. J Bacteriol. 1976 Apr;126(1):122–131. doi: 10.1128/jb.126.1.122-131.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maciag I. E., Viret J. F., Alonso J. C. Replication and incompatibility properties of plasmid pUB110 in Bacillus subtilis. Mol Gen Genet. 1988 May;212(2):232–240. doi: 10.1007/BF00334690. [DOI] [PubMed] [Google Scholar]
- Magnusson K., Hederstedt L., Rutberg L. Cloning and expression in Escherichia coli of sdhA, the structural gene for cytochrome b558 of the Bacillus subtilis succinate dehydrogenase complex. J Bacteriol. 1985 Jun;162(3):1180–1185. doi: 10.1128/jb.162.3.1180-1185.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandel M., Higa A. Calcium-dependent bacteriophage DNA infection. J Mol Biol. 1970 Oct 14;53(1):159–162. doi: 10.1016/0022-2836(70)90051-3. [DOI] [PubMed] [Google Scholar]
- May B. K., Borthwick I. A., Srivastava G., Pirola B. A., Elliott W. H. Control of 5-aminolevulinate synthase in animals. Curr Top Cell Regul. 1986;28:233–262. doi: 10.1016/b978-0-12-152828-7.50008-1. [DOI] [PubMed] [Google Scholar]
- McClung C. R., Somerville J. E., Guerinot M. L., Chelm B. K. Structure of the Bradyrhizobium japonicum gene hemA encoding 5-aminolevulinic acid synthase. Gene. 1987;54(1):133–139. doi: 10.1016/0378-1119(87)90355-6. [DOI] [PubMed] [Google Scholar]
- McConville M. L., Charles H. P. Isolation of haemin-requiring mutants of Escherichia coli K12. J Gen Microbiol. 1979 Jul;113(1):155–164. doi: 10.1099/00221287-113-1-155. [DOI] [PubMed] [Google Scholar]
- Miczák A., Prágai B., Berek I. Mapping the uroporphyrinogen III cosynthase locus in Bacillus subtilis. Mol Gen Genet. 1979 Jul 24;174(3):293–295. doi: 10.1007/BF00267802. [DOI] [PubMed] [Google Scholar]
- Myers A. M., Crivellone M. D., Koerner T. J., Tzagoloff A. Characterization of the yeast HEM2 gene and transcriptional regulation of COX5 and COR1 by heme. J Biol Chem. 1987 Dec 15;262(35):16822–16829. [PubMed] [Google Scholar]
- Neville D. M., Jr Molecular weight determination of protein-dodecyl sulfate complexes by gel electrophoresis in a discontinuous buffer system. J Biol Chem. 1971 Oct 25;246(20):6328–6334. [PubMed] [Google Scholar]
- Niaudet B., Goze A., Ehrlich S. D. Insertional mutagenesis in Bacillus subtilis: mechanism and use in gene cloning. Gene. 1982 Oct;19(3):277–284. doi: 10.1016/0378-1119(82)90017-8. [DOI] [PubMed] [Google Scholar]
- O'Neill G. P., Chen M. W., Söll D. delta-Aminolevulinic acid biosynthesis in Escherichia coli and Bacillus subtilis involves formation of glutamyl-tRNA. FEMS Microbiol Lett. 1989 Aug;51(3):255–259. doi: 10.1016/0378-1097(89)90406-0. [DOI] [PubMed] [Google Scholar]
- Oh-hama T., Stolowich N. J., Scott A. I. 5-Aminolevulinic acid formation from glutamate via the C5 pathway in Clostridium thermoaceticum. FEBS Lett. 1988 Feb 8;228(1):89–93. doi: 10.1016/0014-5793(88)80591-x. [DOI] [PubMed] [Google Scholar]
- Powell K. A., Cox R., McConville M., Charles H. P. Mutations affecting porphyrin biosynthesis in Escherichia coli. Enzyme. 1973;16(1):65–73. doi: 10.1159/000459363. [DOI] [PubMed] [Google Scholar]
- Raich N., Romeo P. H., Dubart A., Beaupain D., Cohen-Solal M., Goossens M. Molecular cloning and complete primary sequence of human erythrocyte porphobilinogen deaminase. Nucleic Acids Res. 1986 Aug 11;14(15):5955–5968. doi: 10.1093/nar/14.15.5955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasarman A., Nepveu A., Echelard Y., Dymetryszyn J., Drolet M., Goyer C. Molecular cloning and sequencing of the hemD gene of Escherichia coli K-12 and preliminary data on the Uro operon. J Bacteriol. 1987 Sep;169(9):4257–4262. doi: 10.1128/jb.169.9.4257-4262.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenhaut D. S., Curtis P. J. Nucleotide sequence of mouse 5-aminolevulinic acid synthase cDNA and expression of its gene in hepatic and erythroid tissues. Gene. 1986;48(1):55–63. doi: 10.1016/0378-1119(86)90351-3. [DOI] [PubMed] [Google Scholar]
- Scott A. I., Roessner C. A., Stolowich N. J., Karuso P., Williams H. J., Grant S. K., Gonzalez M. D., Hoshino T. Site-directed mutagenesis and high-resolution NMR spectroscopy of the active site of porphobilinogen deaminase. Biochemistry. 1988 Oct 18;27(21):7984–7990. doi: 10.1021/bi00421a002. [DOI] [PubMed] [Google Scholar]
- Spizizen J. TRANSFORMATION OF BIOCHEMICALLY DEFICIENT STRAINS OF BACILLUS SUBTILIS BY DEOXYRIBONUCLEATE. Proc Natl Acad Sci U S A. 1958 Oct 15;44(10):1072–1078. doi: 10.1073/pnas.44.10.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava G., Borthwick I. A., Maguire D. J., Elferink C. J., Bawden M. J., Mercer J. F., May B. K. Regulation of 5-aminolevulinate synthase mRNA in different rat tissues. J Biol Chem. 1988 Apr 15;263(11):5202–5209. [PubMed] [Google Scholar]
- Săsărman A., Chartrand P., Lavoie M., Tardif D., Proschek R., Lapointe C. Mapping of a new hem gene in Escherichia coli K12. J Gen Microbiol. 1979 Aug;113(2):297–303. doi: 10.1099/00221287-113-2-297. [DOI] [PubMed] [Google Scholar]
- Săsărman A., Sanderson K. E., Surdeanu M., Sonea S. Hemin-deficient mutants of Salmonella typhimurium. J Bacteriol. 1970 May;102(2):531–536. doi: 10.1128/jb.102.2.531-536.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Săsărman A., Surdeanu M., Horodniceanu T. Locus determining the synthesis of delta-aminolevulinic acid in Escherichia coli K-12. J Bacteriol. 1968 Nov;96(5):1882–1884. doi: 10.1128/jb.96.5.1882-1884.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai T. N., Moore M. D., Kaplan S. Cloning and characterization of the 5-aminolevulinate synthase gene(s) from Rhodobacter sphaeroides. Gene. 1988 Oct 15;70(1):139–151. doi: 10.1016/0378-1119(88)90112-6. [DOI] [PubMed] [Google Scholar]
- Thomas S. D., Jordan P. M. Nucleotide sequence of the hemC locus encoding porphobilinogen deaminase of Escherichia coli K12. Nucleic Acids Res. 1986 Aug 11;14(15):6215–6226. doi: 10.1093/nar/14.15.6215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tien W., White D. C. Linear sequential arrangement of genes for the biosynthetic pathway of protoheme in Staphylococcus aureus. Proc Natl Acad Sci U S A. 1968 Dec;61(4):1392–1398. doi: 10.1073/pnas.61.4.1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tochikubo K. Changes in terminal respiratory pathways of Bacillus subtilis during germination, outgrowth and vegetative growth. J Bacteriol. 1971 Nov;108(2):652–661. doi: 10.1128/jb.108.2.652-661.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban-Grimal D., Volland C., Garnier T., Dehoux P., Labbe-Bois R. The nucleotide sequence of the HEM1 gene and evidence for a precursor form of the mitochondrial 5-aminolevulinate synthase in Saccharomyces cerevisiae. Eur J Biochem. 1986 May 2;156(3):511–519. doi: 10.1111/j.1432-1033.1986.tb09610.x. [DOI] [PubMed] [Google Scholar]
- Verkamp E., Chelm B. K. Isolation, nucleotide sequence, and preliminary characterization of the Escherichia coli K-12 hemA gene. J Bacteriol. 1989 Sep;171(9):4728–4735. doi: 10.1128/jb.171.9.4728-4735.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetmur J. G., Bishop D. F., Cantelmo C., Desnick R. J. Human delta-aminolevulinate dehydratase: nucleotide sequence of a full-length cDNA clone. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7703–7707. doi: 10.1073/pnas.83.20.7703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierenga R. K., Terpstra P., Hol W. G. Prediction of the occurrence of the ADP-binding beta alpha beta-fold in proteins, using an amino acid sequence fingerprint. J Mol Biol. 1986 Jan 5;187(1):101–107. doi: 10.1016/0022-2836(86)90409-2. [DOI] [PubMed] [Google Scholar]
- Woese C. R. Bacterial evolution. Microbiol Rev. 1987 Jun;51(2):221–271. doi: 10.1128/mr.51.2.221-271.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]