Abstract
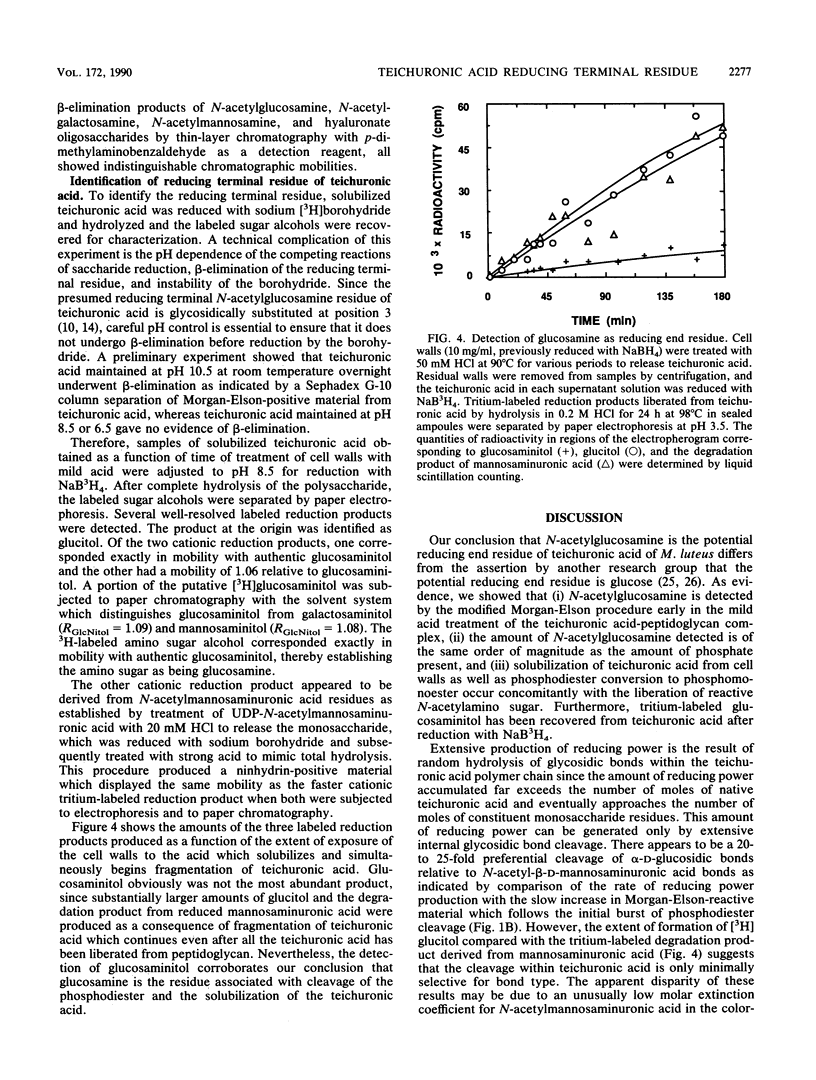
Teichuronic acid-peptidoglycan complex isolated from Micrococcus luteus cells by lysozyme digestion in osmotically stabilized medium was treated with mild acid to cleave the linkage joining teichuronic acid to peptidoglycan. This labile linkage was shown to be the phosphodiester which joins N-acetylglucosamine, the residue located at the reducing end of the teichuronic acid, through its anomeric hydroxyl group to a 6-phosphomuramic acid, a residue of the glycan strand of peptidoglycan. 31P nuclear magnetic resonance spectroscopy of the lysozyme digest of cell walls demonstrated the presence of a phosphodiester which was converted to a phosphomonoester by the conditions which released teichuronic acid from cell walls. Reduction of acid-liberated reducing end groups by NaB3H4 followed by complete acid hydrolysis yielded [3H] glucosaminitol from the true reducing end residue of teichuronic acid and [3H]glucitol from the sites of fragmentation of teichuronic acid. The amount of N-acetylglucosamine detected was approximately stoichiometric with the amount of phosphate in the complex. Partial fragmentation of teichuronic acid provides an explanation of the previous erroneous identification of the reducing end residue.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BITTER T., MUIR H. M. A modified uronic acid carbazole reaction. Anal Biochem. 1962 Oct;4:330–334. doi: 10.1016/0003-2697(62)90095-7. [DOI] [PubMed] [Google Scholar]
- Campbell J. N., Leyh-Bouille M., Ghuysen J. M. Characterization of the Micrococcus lysodeikticus type of peptidoglycan in walls of other Micrococcaceae. Biochemistry. 1969 Jan;8(1):193–200. doi: 10.1021/bi00829a028. [DOI] [PubMed] [Google Scholar]
- Coley J., Tarelli E., Archibald A. R., Baddiley J. The linkage between teichoic acid and peptidoglycan in bacterial cell walls. FEBS Lett. 1978 Apr 1;88(1):1–9. doi: 10.1016/0014-5793(78)80594-8. [DOI] [PubMed] [Google Scholar]
- Enghofer E., Kress H. An evaluation of the Morgan--Elson assay for 2-amino-2-deoxy sugars. Carbohydr Res. 1979 Nov;76:233–238. doi: 10.1016/0008-6215(79)80022-1. [DOI] [PubMed] [Google Scholar]
- Hase S., Matsushima Y. Structural studies on a glucose containing polysaccharide obtained from Micrococcus lysodeikticus cell walls. I. J Biochem. 1970 Nov;68(5):723–730. doi: 10.1093/oxfordjournals.jbchem.a129406. [DOI] [PubMed] [Google Scholar]
- Hase S., Matsushima Y. Structural studies on a glucose-containing polysaccharide obtained from cell walls of Micrococcus lysodeikticus. 3. Determination of the structure. J Biochem. 1972 Nov;72(5):1117–1128. doi: 10.1093/oxfordjournals.jbchem.a129999. [DOI] [PubMed] [Google Scholar]
- Hase S., Matsushima Y. The structure of the branching point between acidic polysaccharide and peptidoglycan in Micrococcus lysodeikticus cell wall. J Biochem. 1977 May;81(5):1181–1186. [PubMed] [Google Scholar]
- Hughes R. C. The cell wall of Bacillus licheniformis N.C.T.C. 6346. Linkage between the teichuronic acid and mucopeptide components. Biochem J. 1970 Apr;117(3):431–439. doi: 10.1042/bj1170431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imanaga Y., Park J. T. Studies on the cell walls of Micrococcus lysodeikticus. Fractionation of the nondialyzable components from a lysozyme digest of cell walls. Biochemistry. 1972 Oct 10;11(21):4006–4012. doi: 10.1021/bi00771a026. [DOI] [PubMed] [Google Scholar]
- Jeanloz R. W. The chemical structure of the cell wall of gram-positive bacteria. Pure Appl Chem. 1967;14(1):57–70. doi: 10.1351/pac196714010057. [DOI] [PubMed] [Google Scholar]
- Johnson G. L., Hoger J. H., Ratnayake J. H., Anderson J. S. Characterization of three intermediates in the biosynthesis of teichuronic acid of Micrococcus luteus. Arch Biochem Biophys. 1984 Dec;235(2):679–691. doi: 10.1016/0003-9861(84)90244-3. [DOI] [PubMed] [Google Scholar]
- Johnson S. D., Lacher K. P., Anderson J. S. Carbon-13 nuclear magnetic resonance spectroscopic study of teichuronic acid from Micrococcus luteus cell walls. Comparison of the polysaccharide isolated from cells with that synthesized in vitro. Biochemistry. 1981 Aug 4;20(16):4781–4785. doi: 10.1021/bi00519a039. [DOI] [PubMed] [Google Scholar]
- Jürgens U. J., Weckesser J. Polysaccharide covalently linked to the peptidoglycan of the cyanobacterium Synechocystis sp. strain PCC6714. J Bacteriol. 1986 Nov;168(2):568–573. doi: 10.1128/jb.168.2.568-573.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanetsuna F., Blas G. S. Chemical analysis of a mycolic acid-arabinogalactan-mucopeptide complex of mycobacterial cell wall. Biochim Biophys Acta. 1970 Jun;208(3):434–443. doi: 10.1016/0304-4165(70)90216-3. [DOI] [PubMed] [Google Scholar]
- Kato K., Iwata S., Matsuda T., Kotani S. Isolation of glucosamine 6-phosphate from the cell walls of Micrococcus lysodeikticus. Biken J. 1978 Jun;21(2):63–67. [PubMed] [Google Scholar]
- Kaya S., Araki Y., Ito E. Structural studies on the linkage unit between poly(galactosylglycerol phosphate) and peptidoglycan in cell walls of Bacillus coagulans. Eur J Biochem. 1985 Feb 15;147(1):41–46. doi: 10.1111/j.1432-1033.1985.tb08715.x. [DOI] [PubMed] [Google Scholar]
- Kaya S., Yokoyama K., Araki Y., Ito E. N-acetylmannosaminyl(1----4)N-acetylglucosamine, a linkage unit between glycerol teichoic acid and peptidoglycan in cell walls of several Bacillus strains. J Bacteriol. 1984 Jun;158(3):990–996. doi: 10.1128/jb.158.3.990-996.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima N., Araki Y., Ito E. Structure of the linkage units between ribitol teichoic acids and peptidoglycan. J Bacteriol. 1985 Jan;161(1):299–306. doi: 10.1128/jb.161.1.299-306.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima N., Uchikawa K., Araki Y., Ito E. A common linkage saccharide unit between teichoic acids and peptidoglycan in cell walls of Bacillus coagulans. J Biochem. 1985 Apr;97(4):1085–1092. doi: 10.1093/oxfordjournals.jbchem.a135152. [DOI] [PubMed] [Google Scholar]
- Kojima N., Uchikawa K., Araki Y., Ito E. Structural studies on the minor teichoic acid of Bacillus coagulans AHU 1631. Eur J Biochem. 1986 Mar 17;155(3):521–526. doi: 10.1111/j.1432-1033.1986.tb09519.x. [DOI] [PubMed] [Google Scholar]
- Liu T. Y., Gotschlich E. C. Muramic acid phosphate as a component of the mucopeptide of Gram-positive bacteria. J Biol Chem. 1967 Feb 10;242(3):471–476. [PubMed] [Google Scholar]
- Nasir-ud-Din, Lhermitte M., Lamblin G., Jeanloz R. W. The phosphate diester linkage of the peptidoglycan polysaccharide moieties of Micrococcus lysodeikticus cell wall. J Biol Chem. 1985 Aug 25;260(18):9981–9987. [PubMed] [Google Scholar]
- Owen P., Freer J. H. Isolation and properties of mesosomal membrane fractions from Micrococcus lysodeikticus. Biochem J. 1972 Oct;129(4):907–917. doi: 10.1042/bj1290907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PERKINS H. R. A polymer containing glucose and aminohexuronic acid isolated from the cell walls of micrococcus lysodeikticus. Biochem J. 1963 Mar;86:475–483. doi: 10.1042/bj0860475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REISSIG J. L., STORMINGER J. L., LELOIR L. F. A modified colorimetric method for the estimation of N-acetylamino sugars. J Biol Chem. 1955 Dec;217(2):959–966. [PubMed] [Google Scholar]
- Rohr T. E., Levy G. N., Stark N. J., Anderson J. S. Initial reactions in biosynthesis of teichuronic acid of Micrococcus lysodeikticus cell walls. J Biol Chem. 1977 May 25;252(10):3460–3465. [PubMed] [Google Scholar]
- Sharon N., Jeanloz R. W. A procedure for the preparation of gram-quantities of bacterial cell walls. Experientia. 1964 May 15;20(5):253–254. doi: 10.1007/BF02151786. [DOI] [PubMed] [Google Scholar]
- Yamazaki T., Laske D. W., Herscovics A., Warren C. D., Jeanloz R. W. Biosynthesis of a D-glucosyl polyisoprenyl diphosphate in particulate preparations of Micrococcus lysodeikticus. Carbohydr Res. 1983 Aug 16;120:159–170. doi: 10.1016/0008-6215(83)88014-8. [DOI] [PubMed] [Google Scholar]


