Abstract
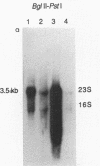
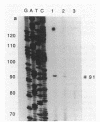
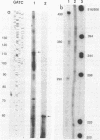
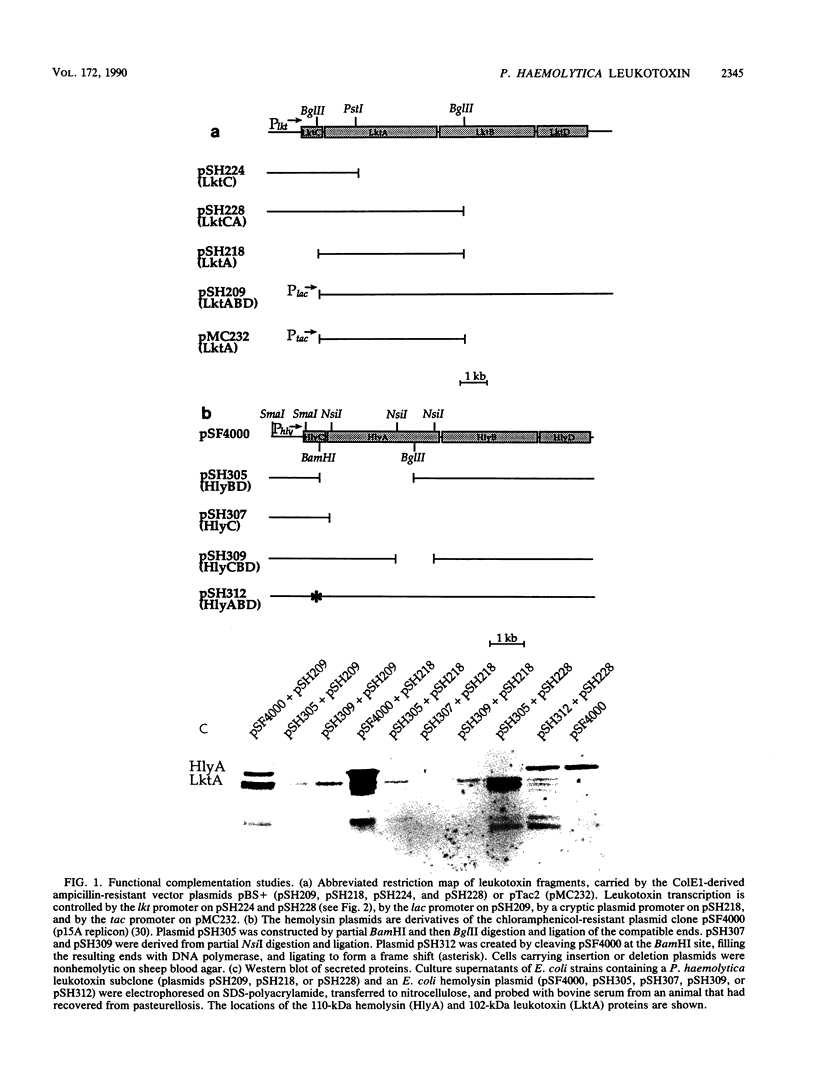
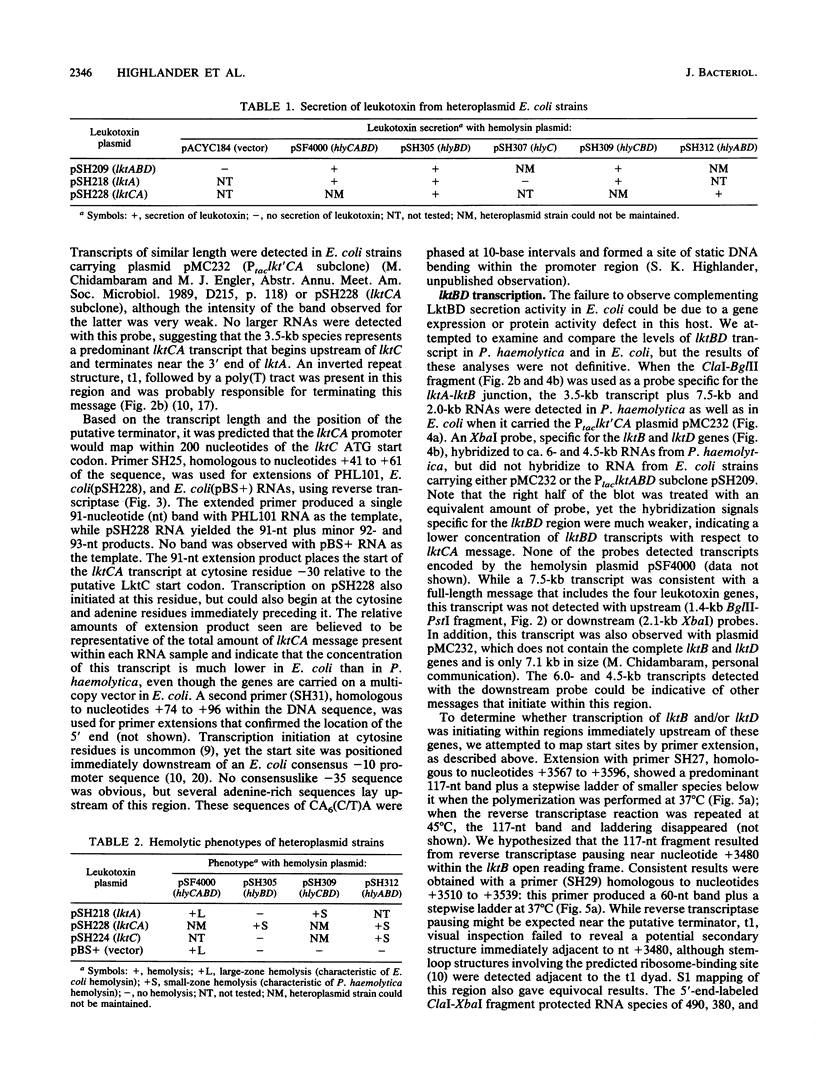
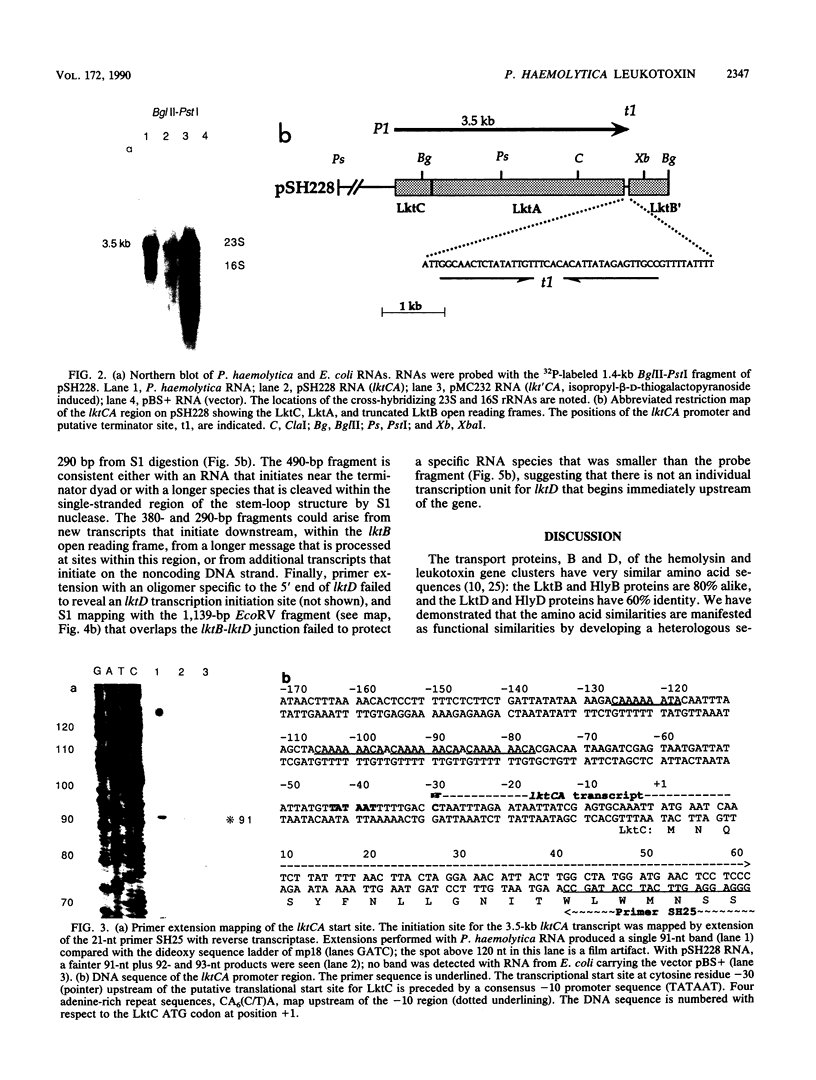
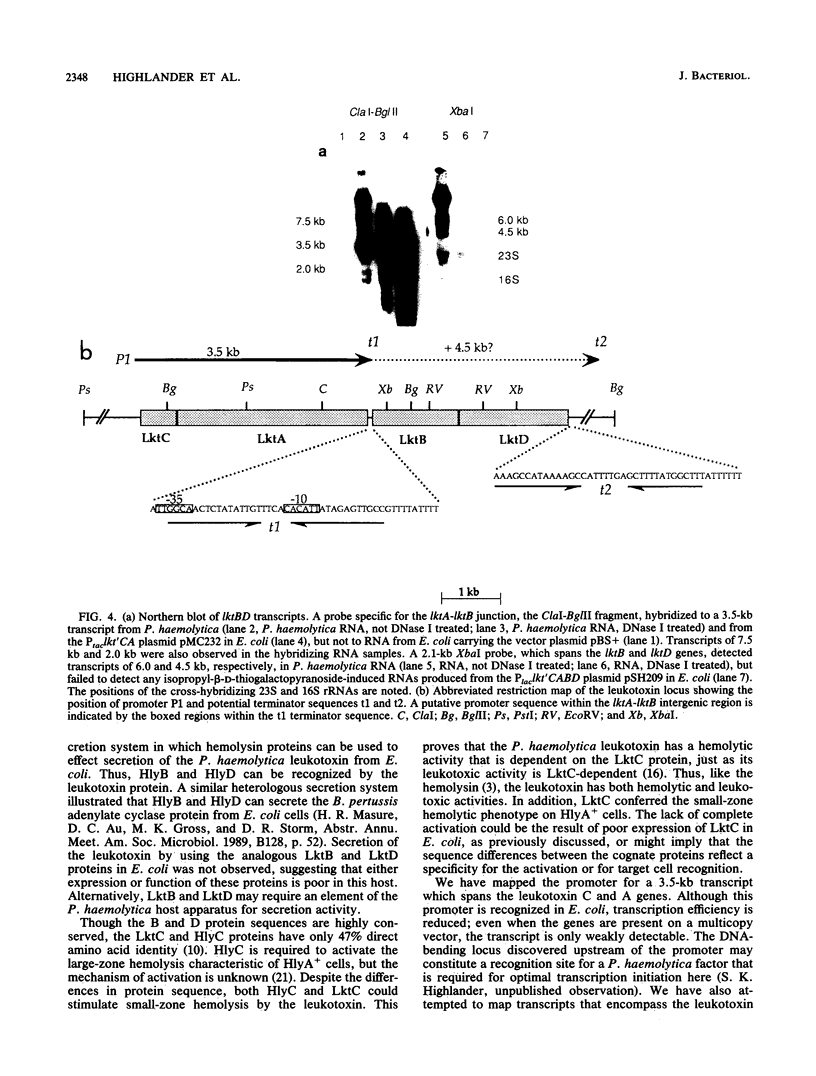
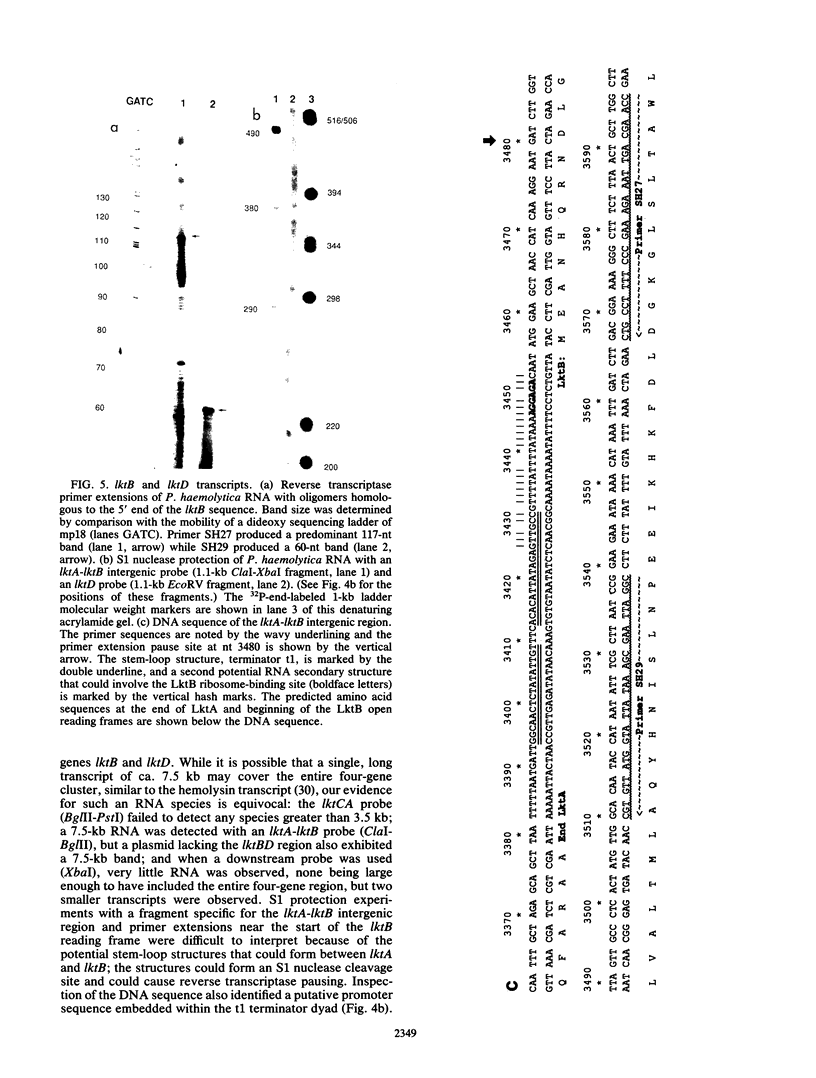
The Pasteurella haemolytica leukotoxin gene cluster (lktCABD) is homologous to the Escherichia coli hemolysin locus (hlyCABD). Since the cloned leukotoxin (LktA) is not secreted from E. coli cells, a heteroplasmid complementation system was developed that permits secretion of the leukotoxin from cells expressing the hemolysin transport proteins HlyB and HlyD. We observed that the secreted leukotoxin protein had weak hemolytic activity when activated by either the HlyC or LktC proteins and that LktC expressed in E. coli could confer weak hemolytic activity upon hemolysin. Thus, it appears that the accessory proteins of the leukotoxin and hemolysin gene clusters are functionally similar, although their expression in E. coli is not equivalent. Northern (RNA) blot analysis of the P. haemolytica leukotoxin gene cluster revealed a major 3.5-kilobase transcript that includes the lktC and lktA genes. The start site for this transcript mapped to a cytosine residue 30 nucleotides upstream from the putative start of lktC; a similar initiation site was observed in E. coli, although adjacent cytosine and adenine residues were also utilized. The 3.5-kilobase transcript terminated near the rho-independent terminator structure between lktA and lktB, but transcription may continue, via antitermination or de novo transcription initiation, into the downstream lktB and lktD genes. We propose that the lack of LktB and LktD function in E. coli is a result, at least in part, of poor lktBD transcription and suggest that a P. haemolytica-specific regulator is required for optimal expression of the leukotoxin genes.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baluyut C. S., Simonson R. R., Bemrick W. J., Maheswaran S. K. Interaction of Pasteurella haemolytica with bovine neutrophils: identification and partial characterization of a cytotoxin. Am J Vet Res. 1981 Nov;42(11):1920–1926. [PubMed] [Google Scholar]
- Cavalieri S. J., Bohach G. A., Snyder I. S. Escherichia coli alpha-hemolysin: characteristics and probable role in pathogenicity. Microbiol Rev. 1984 Dec;48(4):326–343. doi: 10.1128/mr.48.4.326-343.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Y. F., Young R., Post D., Struck D. K. Identification and characterization of the Pasteurella haemolytica leukotoxin. Infect Immun. 1987 Oct;55(10):2348–2354. doi: 10.1128/iai.55.10.2348-2354.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Confer A. W., Panciera R. J., Corstvet R. E., Rummage J. A., Fulton R. W. Bovine pneumonic pasteurellosis: effect of culture age of Pasteurella haemolytica used as a live vaccine. Am J Vet Res. 1984 Dec;45(12):2543–2545. [PubMed] [Google Scholar]
- Felmlee T., Pellett S., Welch R. A. Nucleotide sequence of an Escherichia coli chromosomal hemolysin. J Bacteriol. 1985 Jul;163(1):94–105. doi: 10.1128/jb.163.1.94-105.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filion L. G., Willson P. J., Bielefeldt-Ohmann H., Babiuk L. A., Thomson R. G. The possible role of stress in the induction of pneumonic pasteurellosis. Can J Comp Med. 1984 Jul;48(3):268–274. [PMC free article] [PubMed] [Google Scholar]
- Glaser P., Ladant D., Sezer O., Pichot F., Ullmann A., Danchin A. The calmodulin-sensitive adenylate cyclase of Bordetella pertussis: cloning and expression in Escherichia coli. Mol Microbiol. 1988 Jan;2(1):19–30. [PubMed] [Google Scholar]
- Hawley D. K., McClure W. R. Compilation and analysis of Escherichia coli promoter DNA sequences. Nucleic Acids Res. 1983 Apr 25;11(8):2237–2255. doi: 10.1093/nar/11.8.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Highlander S. K., Chidambaram M., Engler M. J., Weinstock G. M. DNA sequence of the Pasteurella haemolytica leukotoxin gene cluster. DNA. 1989 Jan-Feb;8(1):15–28. doi: 10.1089/dna.1.1989.8.15. [DOI] [PubMed] [Google Scholar]
- Kassavetis G. A., Geiduschek E. P. Bacteriophage T4 late promoters: mapping 5' ends of T4 gene 23 mRNAs. EMBO J. 1982;1(1):107–114. doi: 10.1002/j.1460-2075.1982.tb01132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khandjian E. W. UV crosslinking of RNA to nylon membrane enhances hybridization signals. Mol Biol Rep. 1986;11(2):107–115. doi: 10.1007/BF00364822. [DOI] [PubMed] [Google Scholar]
- Kolodrubetz D., Dailey T., Ebersole J., Kraig E. Cloning and expression of the leukotoxin gene from Actinobacillus actinomycetemcomitans. Infect Immun. 1989 May;57(5):1465–1469. doi: 10.1128/iai.57.5.1465-1469.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koronakis V., Cross M., Hughes C. Expression of the E.coli hemolysin secretion gene hlyB involves transcript anti-termination within the hly operon. Nucleic Acids Res. 1988 Jun 10;16(11):4789–4800. doi: 10.1093/nar/16.11.4789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koronakis V., Cross M., Senior B., Koronakis E., Hughes C. The secreted hemolysins of Proteus mirabilis, Proteus vulgaris, and Morganella morganii are genetically related to each other and to the alpha-hemolysin of Escherichia coli. J Bacteriol. 1987 Apr;169(4):1509–1515. doi: 10.1128/jb.169.4.1509-1515.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo R. Y., Shewen P. E., Strathdee C. A., Greer C. N. Cloning and expression of the leukotoxin gene of Pasteurella haemolytica A1 in Escherichia coli K-12. Infect Immun. 1985 Dec;50(3):667–671. doi: 10.1128/iai.50.3.667-671.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo R. Y., Strathdee C. A., Shewen P. E. Nucleotide sequence of the leukotoxin genes of Pasteurella haemolytica A1. Infect Immun. 1987 Sep;55(9):1987–1996. doi: 10.1128/iai.55.9.1987-1996.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markham R. J., Ramnaraine M. L., Muscoplat C. C. Cytotoxic effect of Pasteurella haemolytica on bovine polymorphonuclear leukocytes and impaired production of chemotactic factors by Pasteurella haemolytica-infected alveolar macrophages. Am J Vet Res. 1982 Feb;43(2):285–288. [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- McClure W. R. Mechanism and control of transcription initiation in prokaryotes. Annu Rev Biochem. 1985;54:171–204. doi: 10.1146/annurev.bi.54.070185.001131. [DOI] [PubMed] [Google Scholar]
- Nicaud J. M., Mackman N., Gray L., Holland I. B. Characterisation of HlyC and mechanism of activation and secretion of haemolysin from E. coli 2001. FEBS Lett. 1985 Aug 5;187(2):339–344. doi: 10.1016/0014-5793(85)81272-2. [DOI] [PubMed] [Google Scholar]
- Peck L. J., Wang J. C. Transcriptional block caused by a negative supercoiling induced structural change in an alternating CG sequence. Cell. 1985 Jan;40(1):129–137. doi: 10.1016/0092-8674(85)90316-2. [DOI] [PubMed] [Google Scholar]
- Shewen P. E., Wilkie B. N. Cytotoxin of Pasteurella haemolytica acting on bovine leukocytes. Infect Immun. 1982 Jan;35(1):91–94. doi: 10.1128/iai.35.1.91-94.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strathdee C. A., Lo R. Y. Cloning, nucleotide sequence, and characterization of genes encoding the secretion function of the Pasteurella haemolytica leukotoxin determinant. J Bacteriol. 1989 Feb;171(2):916–928. doi: 10.1128/jb.171.2.916-928.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strathdee C. A., Lo R. Y. Extensive homology between the leukotoxin of Pasteurella haemolytica A1 and the alpha-hemolysin of Escherichia coli. Infect Immun. 1987 Dec;55(12):3233–3236. doi: 10.1128/iai.55.12.3233-3236.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner W., Vogel M., Goebel W. Transport of hemolysin across the outer membrane of Escherichia coli requires two functions. J Bacteriol. 1983 Apr;154(1):200–210. doi: 10.1128/jb.154.1.200-210.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch R. A., Hull R., Falkow S. Molecular cloning and physical characterization of a chromosomal hemolysin from Escherichia coli. Infect Immun. 1983 Oct;42(1):178–186. doi: 10.1128/iai.42.1.178-186.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch R. A. Identification of two different hemolysin determinants in uropathogenic Proteus isolates. Infect Immun. 1987 Sep;55(9):2183–2190. doi: 10.1128/iai.55.9.2183-2190.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch R. A., Pellett S. Transcriptional organization of the Escherichia coli hemolysin genes. J Bacteriol. 1988 Apr;170(4):1622–1630. doi: 10.1128/jb.170.4.1622-1630.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]