Abstract
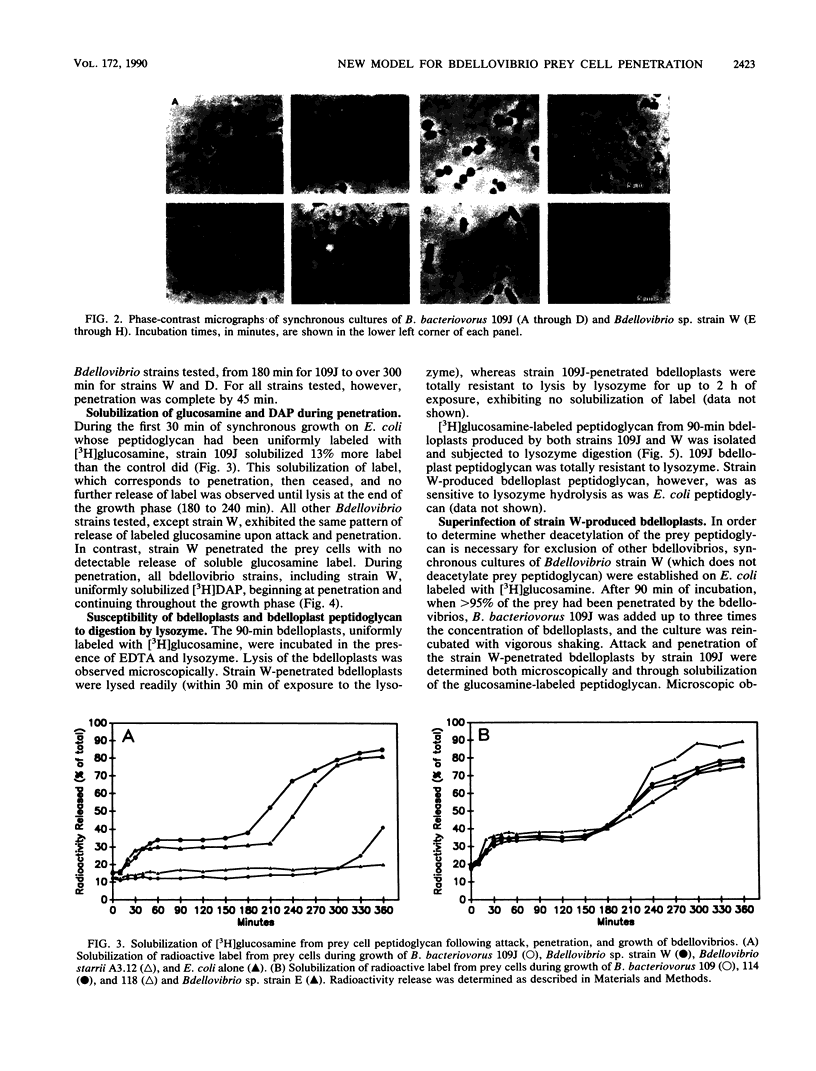
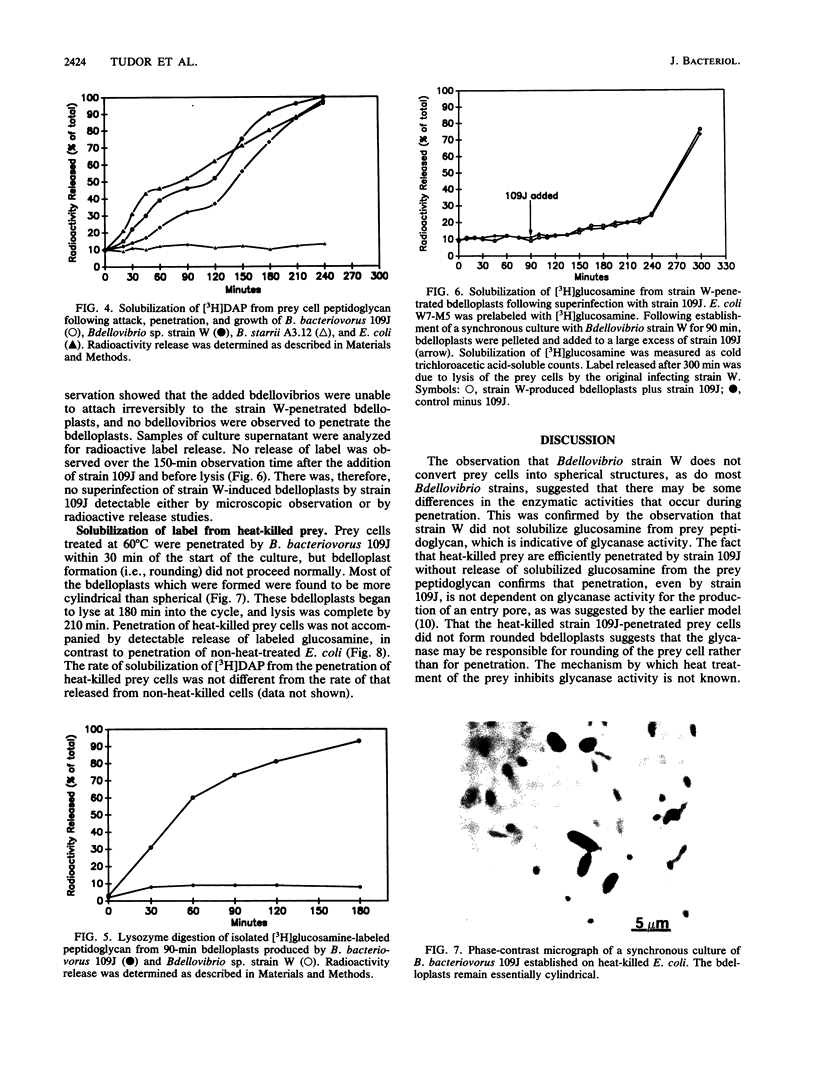
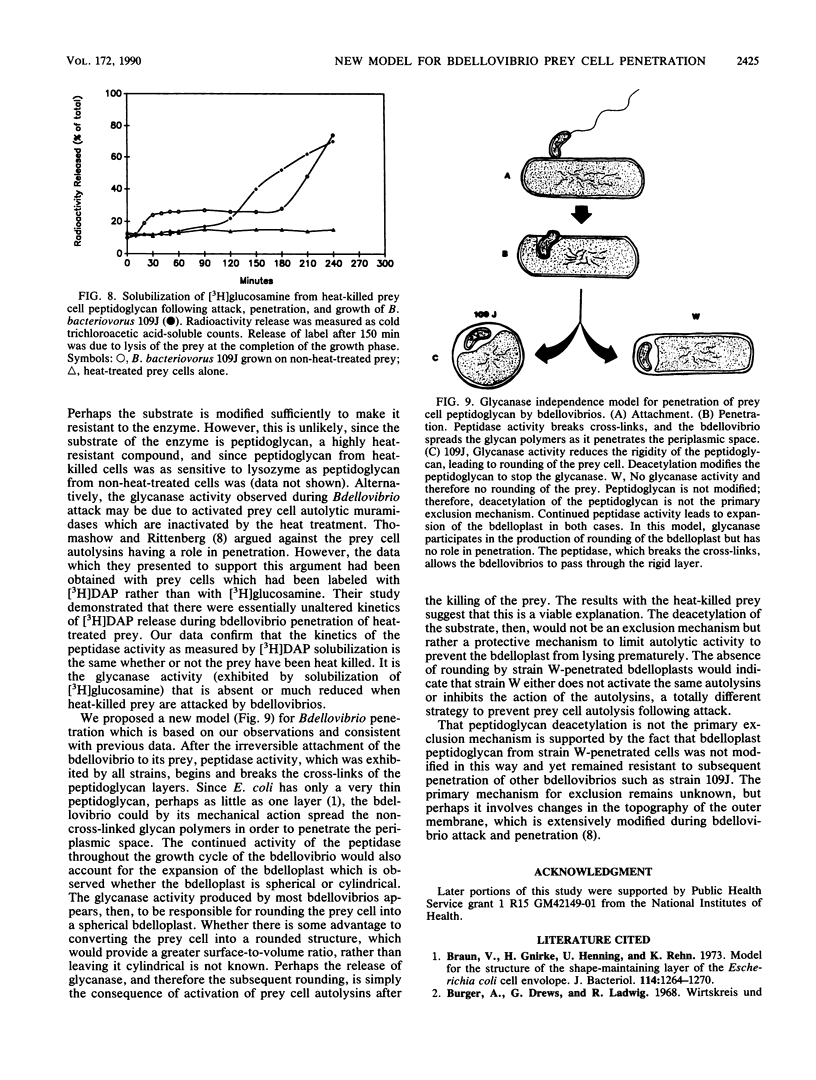
Bdellovibrio bacteriovorus 109J and most other bdellovibrios cause prey cells to round following penetration. Bdellovibrio sp. strain W does not cause rounding of the prey. Analysis of enzyme activities during the early stages of bdellovibrio attack indicated that strain W differs from most other bdellovibrios in that there is no glycanase activity produced during penetration. Likewise, heat-killed prey were penetrated normally by strain 109J, but the resulting bdelloplast did not become round and no glycanase was detected, indicating that glycanase is not essential for penetration. Peptidoglycan from prey cells penetrated by strain W was sensitive to lysozyme, but these cells were not susceptible to attack and penetration by strain 109J, indicating that peptidoglycan deacetylation is not the primary exclusion mechanism. We propose a model in which it is the peptidase activity of the bdellovibrios which allows them to breach the peptidoglycan of their prey and in which the glycanase activity exhibited by strain 109J and other bdellovibrios is responsible for the rounding of the bdelloplast.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Braun V., Gnirke H., Henning U., Rehn K. Model for the structure of the shape-maintaining layer of the Escherichia coli cell envelope. J Bacteriol. 1973 Jun;114(3):1264–1270. doi: 10.1128/jb.114.3.1264-1270.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fackrell H. B., Robinson J. Purification and characterization of a lytic peptidase produced by Bdellovibrio bacteriovorus 6-5-S. Can J Microbiol. 1973 May;19(5):659–666. doi: 10.1139/m73-107. [DOI] [PubMed] [Google Scholar]
- Hespell R. B. Intraperiplasmic growth of Bdellovibrio bacteriovorus on heat-treated Escherichia coli. J Bacteriol. 1978 Mar;133(3):1156–1162. doi: 10.1128/jb.133.3.1156-1162.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J. C., Starr M. P. Possible enzymatic base of bacteriolysis by bdellovibrios. Arch Mikrobiol. 1973 Feb 5;89(2):147–167. doi: 10.1007/BF00425030. [DOI] [PubMed] [Google Scholar]
- Ruby E. G., Rittenberg S. C. Differentiation after premature release of intraperiplasmically growing Bdellovibrio bacteriovorous. J Bacteriol. 1983 Apr;154(1):32–40. doi: 10.1128/jb.154.1.32-40.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shilo M., Bruff B. Lysis of Gram-negative bacteria by host-independent ectoparasitic Bdellovibrio bacteriovorus isolates. J Gen Microbiol. 1965 Sep;40(3):317–328. doi: 10.1099/00221287-40-3-317. [DOI] [PubMed] [Google Scholar]
- Thomashow M. F., Rittenberg S. C. Intraperiplasmic growth of Bdellovibrio bacteriovorus 109J: N-deacetylation of Escherichia coli peptidoglycan amino sugars. J Bacteriol. 1978 Sep;135(3):1008–1014. doi: 10.1128/jb.135.3.1008-1014.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomashow M. F., Rittenberg S. C. Intraperiplasmic growth of Bdellovibrio bacteriovorus 109J: attachment of long-chain fatty acids to escherichia coli peptidoglycan. J Bacteriol. 1978 Sep;135(3):1015–1023. doi: 10.1128/jb.135.3.1015-1023.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomashow M. F., Rittenberg S. C. Intraperiplasmic growth of Bdellovibrio bacteriovorus 109J: solubilization of Escherichia coli peptidoglycan. J Bacteriol. 1978 Sep;135(3):998–1007. doi: 10.1128/jb.135.3.998-1007.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]