Abstract
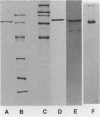
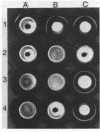
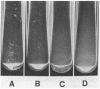
The adhesin of Bacteroides loeschei PK1295 that mediates coaggregation with Streptococcus sanguis 34 and hemagglutination of erythrocytes was purified to electrophoretic homogeneity. The lectinlike protein has an estimated native Mr of 450,000 and consists of six subunits of identical molecular weight (Mr 75,000). The purified adhesin appears to be a basic protein with a pI between 7.4 and 8.0. Amino acid and N-terminal sequence analyses were carried out with the purified protein. These indicated that the protein contains a large number of Asx and Glx residues as well as basic amino acid residues. The binding site of the pure adhesin retained its native configuration during purification. When preincubated with streptococcal partner cells at pH 4.6, the adhesin prevented B. loeschei cells from coaggregating with the streptococci. An adhesin preparation adjusted to a pH of 6.8 rapidly agglutinated both streptococci and neuraminidase-treated erythrocytes. Galactosides inhibited the agglutination reactions.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Batteiger B., Newhall W. J., 5th, Jones R. B. The use of Tween 20 as a blocking agent in the immunological detection of proteins transferred to nitrocellulose membranes. J Immunol Methods. 1982 Dec 30;55(3):297–307. doi: 10.1016/0022-1759(82)90089-8. [DOI] [PubMed] [Google Scholar]
- Brennan M. J., Cisar J. O., Vatter A. E., Sandberg A. L. Lectin-dependent attachment of Actinomyces naeslundii to receptors on epithelial cells. Infect Immun. 1984 Nov;46(2):459–464. doi: 10.1128/iai.46.2.459-464.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan M. J., Joralmon R. A., Cisar J. O., Sandberg A. L. Binding of Actinomyces naeslundii to glycosphingolipids. Infect Immun. 1987 Feb;55(2):487–489. doi: 10.1128/iai.55.2.487-489.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassels F. J., London J. Isolation of a coaggregation-inhibiting cell wall polysaccharide from Streptococcus sanguis H1. J Bacteriol. 1989 Jul;171(7):4019–4025. doi: 10.1128/jb.171.7.4019-4025.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- DUGUID J. P., SMITH I. W., DEMPSTER G., EDMUNDS P. N. Non-flagellar filamentous appendages (fimbriae) and haemagglutinating activity in Bacterium coli. J Pathol Bacteriol. 1955 Oct;70(2):335–348. doi: 10.1002/path.1700700210. [DOI] [PubMed] [Google Scholar]
- Hanson M. S., Hempel J., Brinton C. C., Jr Purification of the Escherichia coli type 1 pilin and minor pilus proteins and partial characterization of the adhesin protein. J Bacteriol. 1988 Aug;170(8):3350–3358. doi: 10.1128/jb.170.8.3350-3358.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoschützky H., Lottspeich F., Jann K. Isolation and characterization of the alpha-galactosyl-1,4-beta-galactosyl-specific adhesin (P adhesin) from fimbriated Escherichia coli. Infect Immun. 1989 Jan;57(1):76–81. doi: 10.1128/iai.57.1.76-81.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones G. W., Isaacson R. E. Proteinaceous bacterial adhesins and their receptors. Crit Rev Microbiol. 1983;10(3):229–260. doi: 10.3109/10408418209113564. [DOI] [PubMed] [Google Scholar]
- King J., Laemmli U. K. Polypeptides of the tail fibres of bacteriophage T4. J Mol Biol. 1971 Dec 28;62(3):465–477. doi: 10.1016/0022-2836(71)90148-3. [DOI] [PubMed] [Google Scholar]
- Klemm P. Fimbrial adhesions of Escherichia coli. Rev Infect Dis. 1985 May-Jun;7(3):321–340. doi: 10.1093/clinids/7.3.321. [DOI] [PubMed] [Google Scholar]
- Kolenbrander P. E. Intergeneric coaggregation among human oral bacteria and ecology of dental plaque. Annu Rev Microbiol. 1988;42:627–656. doi: 10.1146/annurev.mi.42.100188.003211. [DOI] [PubMed] [Google Scholar]
- Lambin P., Fine J. M. Molecular weight estimation of proteins by electrophoresis in linear polyacrylamide gradient gels in the absence of denaturing agents. Anal Biochem. 1979 Sep 15;98(1):160–168. doi: 10.1016/0003-2697(79)90721-8. [DOI] [PubMed] [Google Scholar]
- Lund B., Lindberg F., Marklund B. I., Normark S. The PapG protein is the alpha-D-galactopyranosyl-(1----4)-beta-D-galactopyranose-binding adhesin of uropathogenic Escherichia coli. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5898–5902. doi: 10.1073/pnas.84.16.5898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund B., Lindberg F., Normark S. Structure and antigenic properties of the tip-located P pilus proteins of uropathogenic Escherichia coli. J Bacteriol. 1988 Apr;170(4):1887–1894. doi: 10.1128/jb.170.4.1887-1894.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntire F. C., Vatter A. E., Baros J., Arnold J. Mechanism of coaggregation between Actinomyces viscosus T14V and Streptococcus sanguis 34. Infect Immun. 1978 Sep;21(3):978–988. doi: 10.1128/iai.21.3.978-988.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooi F. R., van Buuren M., Koopman G., Roosendaal B., de Graaf F. K. K88ab gene of Escherichia coli encodes a fimbria-like protein distinct from the K88ab fimbrial adhesin. J Bacteriol. 1984 Aug;159(2):482–487. doi: 10.1128/jb.159.2.482-487.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slater G. G. Stable pattern formation and determination of molecular size by pore-limit electrophoresis. Anal Chem. 1969 Jul;41(8):1039–1041. doi: 10.1021/ac60277a003. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlin B. E., Norgren M., Båga M., Normark S. Adhesion to human cells by Escherichia coli lacking the major subunit of a digalactoside-specific pilus-adhesin. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1800–1804. doi: 10.1073/pnas.82.6.1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss E. I., Kolenbrander P. E., London J., Hand A. R., Andersen R. N. Fimbria-associated proteins of Bacteroides loescheii PK1295 mediate intergeneric coaggregations. J Bacteriol. 1987 Sep;169(9):4215–4222. doi: 10.1128/jb.169.9.4215-4222.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss E. I., London J., Kolenbrander P. E., Andersen R. N. Fimbria-associated adhesin of Bacteroides loeschei that recognizes receptors on procaryotic and eucaryotic cells. Infect Immun. 1989 Sep;57(9):2912–2913. doi: 10.1128/iai.57.9.2912-2913.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss E. I., London J., Kolenbrander P. E., Andersen R. N., Fischler C., Siraganian R. P. Characterization of monoclonal antibodies to fimbria-associated adhesins of Bacteroides loescheii PK1295. Infect Immun. 1988 Jan;56(1):219–224. doi: 10.1128/iai.56.1.219-224.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss E. I., London J., Kolenbrander P. E., Hand A. R., Siraganian R. Localization and enumeration of fimbria-associated adhesins of Bacteroides loescheii. J Bacteriol. 1988 Mar;170(3):1123–1128. doi: 10.1128/jb.170.3.1123-1128.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]