Abstract
Background
Accumulated evidence suggests that hydrogen peroxide (H2O2) generated in cells during insulin stimulation plays an integral role in insulin receptor signal transduction. The role of insulin-induced H2O2 in neuronal insulin receptor activation and the origin of insulin-induced H2O2 in neurons remain unclear. The aim of the present study is to test the following hypotheses (1) whether insulin-induced H2O2 is required for insulin receptor autophosphorylation in neurons, and (2) whether mitochondrial respiratory chain is involved in insulin-stimulated H2O2 production, thus playing an integral role in insulin receptor autophosphorylation in neurons.
Results
Insulin stimulation elicited rapid insulin receptor autophosphorylation accompanied by an increase in H2O2 release from cultured cerebellar granule neurons (CGN). N-acetylcysteine (NAC), a H2O2 scavenger, inhibited both insulin-stimulated H2O2 release and insulin-stimulated autophosphorylation of insulin receptor. Inhibitors of respiratory chain-mediated H2O2 production, malonate and carbonyl cyanide-4-(trifluoromethoxy)-phenylhydrazone (FCCP), inhibited both insulin-stimulated H2O2 release from neurons and insulin-stimulated autophosphorylation of insulin receptor. Dicholine salt of succinic acid, a respiratory substrate, significantly enhanced the effect of suboptimal insulin concentration on the insulin receptor autophosphorylation in CGN.
Conclusion
Results of the present study suggest that insulin-induced H2O2 is required for the enhancement of insulin receptor autophosphorylation in neurons. The mitochondrial respiratory chain is involved in insulin-stimulated H2O2 production, thus playing an integral role in the insulin receptor autophosphorylation in neurons.
Background
Accumulated evidence suggests that hydrogen peroxide (H2O2) generated in cells during insulin stimulation plays an integral role in insulin receptor signal transduction [1-4]. Specific molecular targets of H2O2 identified to date include the insulin receptor kinase [5-7], protein tyrosine phosphatases (PTP) [8-11], and the lipid phosphatase PTEN [12], whose activity is modified via oxidative reactions with H2O2. Two distinct insulin-sensitive cellular H2O2 sources have been identified. A membrane-bound NADPH-oxidase is involved in insulin-induced H2O2 production in adipocytes [13-17] and vascular smooth muscle cells [18,19]. The mitochondrial respiratory chain is implicated in insulin-induced H2O2 generation in liver and heart [20,21]. There are experimental data that insulin-induced reactive oxygen species (ROS) and H2O2 play a role in the activation of insulin signaling in neuroblastomas [12,22]. However, the role of insulin-induced H2O2 in neuronal insulin receptor activation and the origin of insulin-induced H2O2 in neurons remain unclear.
The aim of the present study is to test the following hypotheses (1) whether insulin-induced H2O2 is required for insulin receptor autophosphorylation in neurons, and (2) whether mitochondrial respiratory chain is involved in insulin-stimulated H2O2 production, thus playing an integral role in insulin receptor autophosphorylation in neurons.
Results
Insulin-induced H2O2 is required for the enhancement of the insulin receptor autophosphorylation in neurons
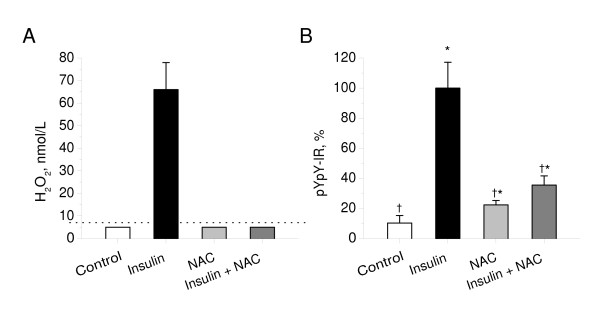
To examine whether insulin stimulates H2O2 production in cultured cerebellar granule neurons (CGN), we measured H2O2 accumulation for 1 min in the incubation medium of CGN cultures, in the absence or presence of insulin. For H2O2 detection, an extremely sensitive assay based on fluorescence of resorufin, a product of a 1:1 stoichiometric reaction of Amplex red dye with H2O2, was used. As shown in Figure 1A, insulin stimulation elicited a marked increase in H2O2 release from CGN to a level of 66 ± 12 nmol/L, although the basal H2O2 release from CGN cultures was below the assay detection limit (< 7 nmol/L).
Figure 1.
Effect of N-acetylcysteine on Insulin-stimulated H2O2 production and the insulin receptor autophosphorylation in cerebellar granule neurons. A: CGN cultures were pre-incubated for 30 min in the absence or presence of N-acetylcysteine (5 mmol/l) in Hepes-buffered salt solution and then exposed to insulin (100 nmol/L). H2O2 release from cultures for 1 min was measured as described in Materials and Methods. Results were normalized by cell density. Columns represent the means ± SD of H2O2 values obtained from five to nine cultures. Dotted line represents a detection limit of the assay (7 nmol/L). B: CGN cultures were pre-incubated for 30 min in the absence or presence of N-acetylcysteine (5 mmol/l) in Hepes-buffered salt solution and then exposed to insulin (100 nmol/L) for 20 min. Autophosphorylation of insulin receptor was measured as described in Materials and Methods. In each experiment, amount of phosphorylated insulin receptor β-subunit (pYpY-IR) was normalized to total amount of insulin receptor β-subunit and expressed as a percentage of the response produced to 100 nmol/L insulin. Columns represent the means ± SD of pYpY-IR values obtained from four to nine culture dishes. *P < 0.05 vs. control.†P < 0.05 vs. insulin.
To determine whether insulin-induced H2O2 is involved in the enhancement of insulin receptor autophosphorylation, we next studied the effects of N-acetylcysteine (NAC), a H2O2 scavenger, on the insulin-stimulated autophosphorylation of the insulin receptor in CGN. As shown in Figure 1A, the pre-incubation of CGN with NAC abolished the insulin-stimulated H2O2 release from cells to undetectable levels (<7 nmol/L), indicating that NAC is a potent scavenger of insulin-induced H2O2 under these experimental conditions. Figure 1B shows that pre-incubation of CGN with NAC resulted in the significant inhibition of insulin-stimulated insulin receptor autophosphorylation (P < 1e-6 vs. insulin). These results suggest that insulin-induced H2O2 is required for the enhancement of insulin receptor autophosphorylation in neurons.
The mitochondrial respiratory chain is involved in insulin-stimulated H2O2 production, thus playing an integral role in the insulin receptor autophosphorylation in neurons
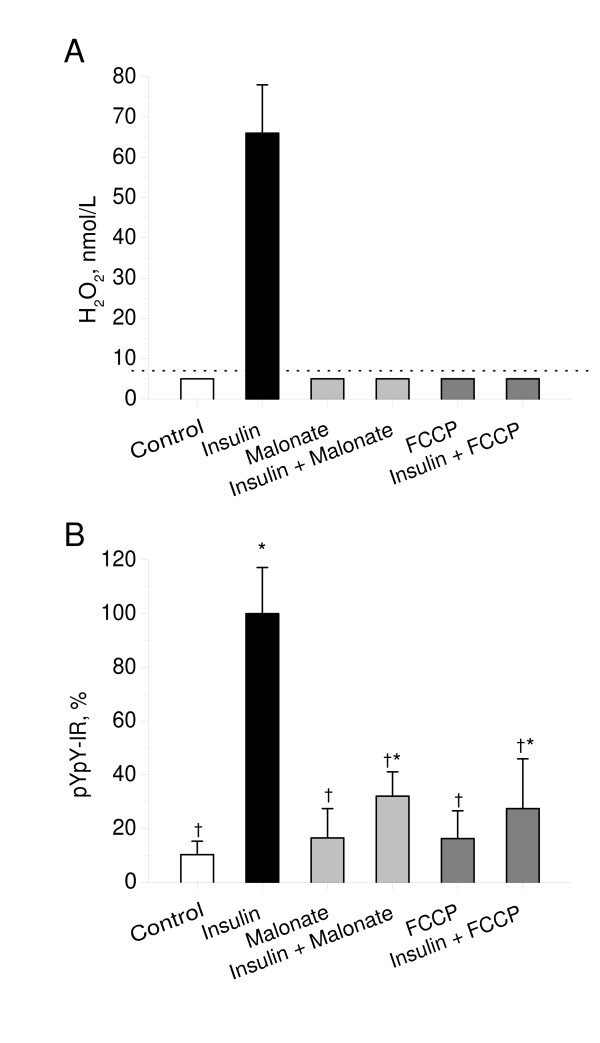
To reveal whether mitochondrial respiratory chain is involved in the insulin-stimulated H2O2 production in neurons, we measured H2O2 release from CGN cultures in the absence or presence of insulin and two inhibitors of respiratory chain-mediated H2O2 production, malonate and carbonyl cyanide-4-(trifluoromethoxy)-phenylhydrazone (FCCP). As shown in Figure 2A, both malonate and FCCP completely abolished insulin-stimulated H2O2 release from CGN. Both malonate and FCCP had no effect on basal H2O2 production in CGN. These data indicate that mitochondrial respiratory chain is involved in insulin-stimulated H2O2 generation in neurons.
Figure 2.
Effects of malonate and FCCP on insulin-stimulated H2O2 production and insulin receptor autophosphorylation in cerebellar granule neurons. A: CGN cultures were pre-incubated for 30 min in Hepes-buffered salt solution and then exposed to insulin (100 nmol/L) in the absence or presence of malonate (2 mmol/L) or FCCP (0.5 μmol/L). H2O2 release from cultures for 1 min was measured as described in Materials and Methods. Results were normalized by cell density. Columns represent the means ± SD of H2O2 values obtained from five to nine cultures. Dotted line represents a detection limit of the assay (7 nmol/L). B: CGN cultures were pre-incubated for 30 min in Hepes-buffered salt solution and then exposed to insulin (100 nmol/L) for 20 min. Malonate (2 mmol/l) or FCCP (0.5 μmol/L) were added to cultures 5 min before the insulin exposure. Autophosphorylation of insulin receptor was measured as described in Materials and Methods. In each experiment, amount of phosphorylated insulin receptor β-subunit (pYpY-IR) was normalized to total amount of insulin receptor β-subunit and expressed as a percentage of the response produced to 100 nmol/L insulin. Columns represent the means ± SD of pYpY-IR values obtained from four to nine culture dishes. *P < 0.05 vs. control.†P < 0.05 vs. insulin.
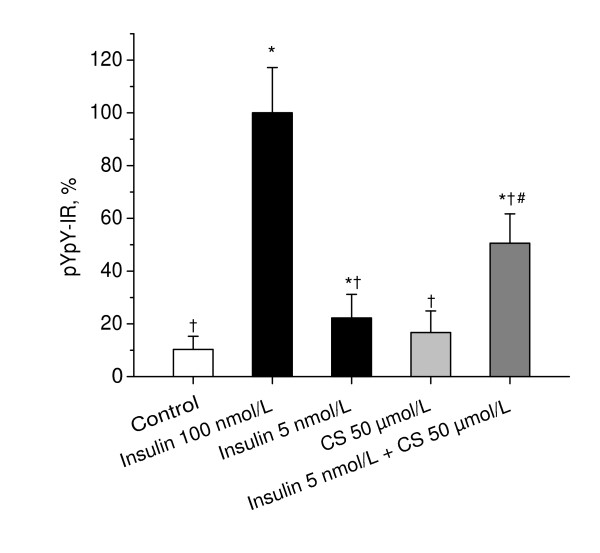
To examine whether mitochondrial respiratory chain is involved in insulin receptor autophosphorylation in neurons, we assessed the effects of inhibitors of respiratory chain-mediated H2O2 production, malonate and FCCP, and a respiratory substrate, dicholine salt of succinic acid (CS), on the insulin-stimulated autophosphorylation of the insulin receptor in primary CGN cultures. Whereas by itself, malonate, FCCP, and CS had no effect on the basal autophosphorylation of the insulin receptor, they significantly influenced the insulin-stimulated autophosphorylation of insulin receptor. As shown in Figure 2B, both malonate and FCCP significantly inhibited the insulin-stimulated autophosphorylation of the insulin receptor (P < 1e-5 vs. insulin). Figure 3 shows that CS significantly enhanced the effect of suboptimal concentration of 5 nmol/L insulin on insulin receptor autophosphorylation (P < 0.001 vs. insulin). These data suggest that mitochondrial respiratory chain plays an integral role in insulin receptor autophosphorylation in neurons.
Figure 3.
Effects of dicholine salt of succinic acid on insulin-stimulated insulin receptor autophosphorylation in cerebellar granule neurons. CGN cultures were pre-incubated for 30 min in Hepes-buffered salt solution and then exposed to insulin (100 nmol/L), insulin (5 nmol/L), CS (50 μmol/L), or a combination of insulin (5 nmol/L) and CS (50 μmol/L) for 20 min. Autophosphorylation of insulin receptor was measured as described in Materials and Methods. In each experiment, amount of phosphorylated insulin receptor β-subunit (pYpY-IR) was normalized to total amount of insulin receptor β-subunit and expressed as a percentage of the response produced to 100 nmol/L insulin. Columns represent the means ± SD of pYpY-IR values obtained from four to nine culture dishes. *P < 0.05 vs. control.†P < 0.05 vs. insulin (100 nmol/L). #P < 0.05 vs. insulin (5 nmol/L).
Discussion
Insulin signaling requires the autophosphorylation of the insulin receptor kinase at tyrosine residues in the activation loop of the kinase domain [23-28]. Upon autophosphorylation, the receptor undergoes a major conformational change resulting in unrestricted access of protein substrates and ATP to the kinase active site and an approximate two-order increase in kinase activity [29-31]. In the present study, we demonstrate that N-acetylcysteine, the H2O2 scavenger, inhibits both insulin-stimulated H2O2 generation and insulin-stimulated insulin receptor autophosphorylation in CGN. These results suggest that insulin-induced H2O2 is required for the enhancement of insulin receptor autophosphorylation in neurons.
We found that mitochondrial respiratory chain is involved in insulin-stimulated H2O2 production, thus playing an integral role in insulin receptor autophosphorylation in neurons. Mitochondrial respiration is a major cellular source of H2O2 that may convert up to 2% of total oxygen consumption into H2O2 in state 4 respiration (oxygen consumption in the absence of ADP) with succinate as a respiratory substrate [32]. Among other respiratory substrates, the complex II substrate succinate provides the highest rates of mitochondrial H2O2 generation in brain mitochondria [33]. Molecular mechanisms of H2O2 production in mitochondria are the subject of intense ongoing research. The respiratory chain reduces oxygen to superoxide anion, which dismutates to H2O2 spontaneously or by the action of superoxide dismutase [34]. Although mitochondria produce H2O2 in all metabolic states, high mitochondrial membrane potential (ΔΨ) characteristic to the resting (State 4) respiration significantly promotes H2O2 generation [33,35]. Substances that decrease (ΔΨ), e.g. malonate and FCCP, inhibit H2O2 generation [33,35-37]. In the present study, we demonstrate that inhibitors of respiratory chain-mediated H2O2 production, malonate and the protonophore FCCP, inhibit both insulin-induced H2O2 generation and insulin-stimulated receptor autophosphorylation in neurons. The respiratory substrate succinate, taken in form of dicholine salt of succinic acid, significantly enhances the stimulatory effect of suboptimal insulin concentration on insulin receptor autophosphorylation. These results, together with our observations that the H2O2 scavenger (NAC) inhibited both insulin-stimulated H2O2 generation and insulin receptor autophosphorylation, suggest that the mitochondrial respiratory chain is involved in insulin-stimulated H2O2 production, thus playing an integral role in insulin receptor autophosphorylation in neurons.
Our prior studies provide evidence that a transient activation of succinate dehydrogenase (SDH) is a mode by which insulin increases the rate of mitochondrial H2O2 generation [20,21]. Earlier, it has been demonstrated that insulin exhibits an immediate stimulatory effect on oxidation of [2,3-14C]-succinate in mitochondrial Krebs cycle, which is almost maximal within 30 sec [38,39]. The results of the present study are consistent with these findings. Malonate, a competitive inhibitor of succinate dehydrogenase, inhibits both insulin-stimulated H2O2 production and the receptor phosphorylation in neurons, indicating a role of SDH in these processes. Although signaling pathways regulating insulin-stimulated SDH activation remain to be elucidated, these pathways seem to be distinct from those induced by autophosphorylated form of insulin receptor. The reason for it is that insulin-stimulated H2O2 burst enhances the autophosphorylation of the insulin receptor, since inhibitors of insulin-stimulated H2O2 generation abolish the receptor autophosphorylation.
A large body of evidence has accumulated that impairments in cerebral insulin receptor signaling may contribute to age-related cognitive decline and Alzheimer's disease [40-43]. In this context, our findings identify mitochondrial respiratory chain as a potential pharmacological target for the treatment of disorders associated with dysfunctional insulin receptor signal transduction in neurons.
Conclusion
Results of the present study suggest that insulin-induced H2O2 is required for the enhancement of insulin receptor autophosphorylation in neurons. The mitochondrial respiratory chain is involved in insulin-stimulated H2O2 production, thus playing an integral role in insulin receptor autophosphorylation in neurons.
Methods
Materials
PhosphoDetect™ Insulin Receptor (pTyr1162/1163) ELISA kit and Insulin Receptor (β-Subunit) ELISA Kit were from Calbiochem. Dicholine salt of succinic acid was prepared by a reaction of succinic acid with choline base in the Russian Scientific Center on Drug Safety (Staraya Kupavna, Moscow region). Other materials were purchased from Sigma, ICN, Gibco, Biosource, Invitrogen, or Acros.
Neuronal culture
Cerebellar granule neurons were prepared from 7- to 8-day-old Wistar rats as described [44,45]. Cerebellum was dissected and placed in ice-cold Ca2+/Mg2+-free Hanks' buffered salt solution (HBSS) without Phenol Red (Gibco). After mincing the tissue with fine scissors, the tissue was placed in Ca2+/Mg2+-free HBSS with Phenol Red and 0.1% trypsin for 15 min at 36°C. Trypsin was inactivated by washing with normal HBSS. Cells were dissociated by trituration and pelleted in HBSS. Then, the cells were resuspended in Neurobasal Medium (Gibco) supplemented with B-27 Supplement (Gibco), 20 mmol/L KCl, GlutaMax (Gibco) and penicillin/streptomycin and plated with density 5 × 106 cells/ml onto 35 mm × 10 mm sterile cell culture dishes which had been previously coated with poly-D-lysine. The cultures were maintained at 36°C in a humidified atmosphere of 5% CO2 and 95% air and fed with supplemented Neurobasal Medium. Cultures were treated on day 3 with 10 μmol/L cytosine arabinoside (Sigma) for 24 h to prevent glial proliferation. Neurons at 7 to 9 days were used for experiments.
Measurement of hydrogen peroxide
H2O2 release from CGN cultures for 1 min was measured fluorimetrically employing the cell-impermeable Amplex Red dye (Invitrogen) in the presence of horseradish peroxidase (Sigma). With Amplex Red, it is possible to perform reliable measurements of H2O2 production by brain mitochondria under physiologically realistic conditions [46]. CGN cultures were pre-incubated for 30 min in Hepes-buffered salt solution (145 mmol/L NaCl, 5.6 mmol/L KCl, 1.8 mmol/L CaCl2, 1 mmol/L MgCl2, 20 mmol/L HEPES, and 5 mmol/L glucose) at pH 7.4 and then exposed to insulin (100 nM) or vehicle. H2O2 release from CGN cultures for 1 min was measured. Where indicated, NAC (5 mmol/L) was added 30 min before the insulin stimulation. Malonate (2 mmol/L) or FCCP (0.5 μmol/L) were added 5 min before the insulin stimulation. In these experiments, the incubation medium was supplemented with 2 μmol/L Amplex Red and 4 IU/ml horseradish peroxidase. Fluorescence was measured with an epifluorescent inverted microscope Axiovert 200 (Carl Zeiss, Germany) equipped with a 20× fluorite objective using excitation at 550 ± 10 nm and fluorescence detection at 610 ± 30 nm. All imaging data were collected and analyzing using the Metafluor 6.1 software (Universal Imaging Corp., USA). Standard curves obtained by adding known amounts of H2O2 to the assay medium were linear up to 1500 nmol/L. Fluorescence values were converted to H2O2 values using these standard curves. The calculated detection limit of the assay was 7 nmol/L. Data were normalized by cell density and expressed as nmol/L H2O2.
Insulin receptor phosphorylation assay
Amounts of double phosphorylated β-subunit of insulin receptor (pYpY-IR) were measured by PhosphoDetect™ insulin receptor (pTyr1162/1163) ELISA kit (Calbiochem) suitable for studies with rat insulin receptor. CGN cultures were pre-incubated for 30 min in Hepes-buffered salt solution (145 mmol/L NaCl, 5.6 mmol/L KCl, 1.8 mmol/L CaCl2, 1 mmol/L MgCl2, 20 mmol/L HEPES, and 5 mmol/L glucose) at pH 7.4 and then exposed to insulin (100 nM) or vehicle for 20 min (the incubation time and the insulin concentration were determined from a time and dose response curves respectively; data not shown). Where indicated, NAC (5 mmol/L) was added 30 min before the insulin stimulation. Malonate (2 mmol/L) or FCCP (0.5 μmol/L) were added 5 min before the insulin stimulation. The experiment was terminated by removing the medium, washing with ice-cold PBS, and adding 120 μL per dish cell lysis buffer (Biosource) supplemented with 1 mmol/L PMSF, 50 mmol/L protease inhibitor set III (Sigma), and 2 mmol/L sodium ortovanadate as the inhibitor of tyrosine phosphatases at 4°C for 20 min. Lysates were centrifuged at 12,000 rpm at 4°C for 12 min. In each CGN lysate, pYpY-IR amounts were measured as described by the manufacturer's manual. Obtained values were normalized to total amounts of insulin receptor β-subunit (IR) measured by insulin receptor (β-subunit) ELISA kit (Calbiochem). The results are expressed as a percentage of the response produced to 100 nmol/L insulin.
Statistics
Data were analyzed for statistical significance by one-way analysis of variance (ANOVA). Values are given as means ± SD. Differences were considered significant at P < 0.05.
Abbreviations
CGN, cerebellar granule neurons; CS, dicholine salt of succinic acid; FCCP, carbonyl cyanide-4-(trifluoromethoxy)-phenylhydrazone; HBSS, Hanks' buffered salt solution; HEPES, 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid; NAC, N-acetylcysteine; PBS, phosphate-buffered saline; PMSF, phenylmethylsulfonyl fluoride; PTEN, phosphatase and tensin homolog; PTPs, protein tyrosine phosphatases; SD, standard deviation; SDH, succinate dehydrogenase.
Authors' contributions
TPS carried out the in vitro studies with CGN cultures and data analysis. YES carried out the in vitro studies with CGN cultures and data analysis. NAP carried out the in vitro studies with CGN cultures and data analysis. VGP participated in the design of the in vitro studies with CGN cultures, critical intellectual discussion, and manuscript evaluation/critique. IAP conceived, designed and coordinated the study, and drafted the manuscript. All authors read and approved the final manuscript.
Acknowledgments
Acknowledgements
This work was funded by Biosignal Ltd., Moscow, Russia.
Contributor Information
Tatiana P Storozhevykh, Email: tstor@mail.ru.
Yana E Senilova, Email: ysenilova@mail.ru.
Nadezhda A Persiyantseva, Email: nadushka99@rambler.ru.
Vsevolod G Pinelis, Email: vpinelis@nczd.ru.
Igor A Pomytkin, Email: ipomytkin@mail.ru.
References
- Mukherjee SP, Lane RH, Lynn WS. Endogenous hydrogen peroxide and peroxidative metabolism in adipocytes in response to insulin and sulfhydryl reagents. Biochem Pharmacol. 1978;27:2589–94. doi: 10.1016/0006-2952(78)90332-5. [DOI] [PubMed] [Google Scholar]
- May JM, de Haen C. Insulin-stimulated intracellular hydrogen peroxide production in rat epididymal fat cells. J Biol Chem. 1979;254:2214–20. [PubMed] [Google Scholar]
- Goldstein BJ, Mahadev K, Wu X, Zhu L, Motoshima H. Role of insulin-induced reactive oxygen species in the insulin signaling pathway. Antioxid Redox Signal. 2005;7:1021–31. doi: 10.1089/ars.2005.7.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein BJ, Mahadev K, Wu X. Redox paradox: insulin action is facilitated by insulin-stimulated reactive oxygen species with multiple potential signaling targets. Diabetes. 2005;54:311–21. doi: 10.2337/diabetes.54.2.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid E, El Benna J, Galter D, Klein G, Droge W. Redox priming of the insulin receptor β-chain associated with altered tyrosine kinase activity and insulin responsiveness in the absence of tyrosine autophosphorylation. FASEB J. 1998;12:863–870. doi: 10.1096/fasebj.12.10.863. [DOI] [PubMed] [Google Scholar]
- Schmid E, Hotz-Wagenblatt A, Hack V, Droge W. Phosphorylation of the insulin receptor kinase by phosphocreatine in combination with hydrogen peroxide: the structural basis of redox priming. FASEB J. 1999;13:1491–1500. doi: 10.1096/fasebj.13.12.1491. [DOI] [PubMed] [Google Scholar]
- Schmitt TL, Hotz-Wagenblatt A, Klein H, Droge W. Interdependent regulation of insulin receptor kinase activity by ADP and hydrogen peroxide. J Biol Chem. 2005;280:3795–3801. doi: 10.1074/jbc.M410352200. [DOI] [PubMed] [Google Scholar]
- Mahadev K, Zilbering A, Zhu L, Goldstein BJ. Insulin-stimulated hydrogen peroxide reversibly inhibits protein-tyrosine phosphatase 1b in vivo and enhances the early insulin action cascade. J Biol Chem. 2001;276:21938–42. doi: 10.1074/jbc.C100109200. [DOI] [PubMed] [Google Scholar]
- Mahadev K, Wu X, Zilbering A, Zhu L, Lawrence JT, Goldstein BJ. Hydrogen peroxide generated during cellular insulin stimulation is integral to activation of the distal insulin signaling cascade in 3T3-L1 adipocytes. J Biol Chem. 2001;276:48662–9. doi: 10.1074/jbc.M105061200. [DOI] [PubMed] [Google Scholar]
- Meng T-C, Buckley DA, Galic S, Tiganis T, Tonks NK. Regulation of insulin signaling through reversible oxidation of the protein-tyrosine phosphatases TC45 and PTP1B. J Biol Chem. 2004;279:37716–37725. doi: 10.1074/jbc.M404606200. [DOI] [PubMed] [Google Scholar]
- Wu X, Zhu L, Zilbering A, Mahadev K, Motoshima H, Yao J, Goldstein BJ. Hyperglycemia potentiates H(2)O(2) production in adipocytes and enhances insulin signal transduction: potential role for oxidative inhibition of thiol-sensitive protein-tyrosine phosphatases. Antioxid Redox Signal. 2005;7:526–37. doi: 10.1089/ars.2005.7.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo JH, Ahn Y, Lee SR, Yeol Yeo C, Chung Hur K. The major target of the endogenously generated reactive oxygen species in response to insulin stimulation is phosphatase and tensin homolog and not phosphoinositide-3 kinase (PI-3 kinase) in the PI-3 kinase/Akt pathway. Mol Biol Cell. 2005;16:348–57. doi: 10.1091/mbc.E04-05-0369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee SP, Lynn WS. Reduced nicotinamide adenine dinucleotide phosphate oxidase in adipocyte plasma membrane and its activation by insulin. Possible role in the hormone's effects on adenylate cyclase and the hexose monophosphate shunt. Arch Biochem Biophys. 1977;184:69–76. doi: 10.1016/0003-9861(77)90327-7. [DOI] [PubMed] [Google Scholar]
- Krieger-Brauer HI, Kather H. Human fat cells possess a plasma membrane-bound H2O2-generating system that is activated by insulin via a mechanism bypassing the receptor kinase. J Clin Invest. 1992;89:1006–13. doi: 10.1172/JCI115641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieger-Brauer HI, Medda PK, Kather H. Insulin-induced activation of NADPH-dependent H2O2 generation in human adipocyte plasma membranes is mediated by Galphai2. J Biol Chem. 1997;272:10135–43. doi: 10.1074/jbc.272.15.10135. [DOI] [PubMed] [Google Scholar]
- Kreuzer J, Nurnberg B, Krieger-Brauer HI. Ligand-dependent autophosphorylation of the insulin receptor is positively regulated by Gi-proteins. Biochem J. 2004;380:831–6. doi: 10.1042/BJ20031659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahadev K, Motoshima H, Wu X, Ruddy JM, Arnold RS, Cheng G, Lambeth JD, Goldstein BJ. The NAD(P)H oxidase homolog Nox4 modulates insulin-stimulated generation of H2O2 and plays an integral role in insulin signal transduction. Mol Cell Biol. 2004;24:1844–54. doi: 10.1128/MCB.24.5.1844-1854.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Yang Y, Zhang S, Kahn AM. Insulin-stimulated hydrogen peroxide increases guanylate cyclase activity in vascular smooth muscle. Hypertension. 2003;42:569–73. doi: 10.1161/01.HYP.0000092441.27668.BD. [DOI] [PubMed] [Google Scholar]
- Yang M, Foster E, Kahn AM. Insulin-stimulated NAD(P)H oxidase activity increases migration of cultured vascular smooth muscle cells. Am J Hypertens. 2005;18:1329–34. doi: 10.1016/j.amjhyper.2005.04.012. [DOI] [PubMed] [Google Scholar]
- Pomytkin IA, Kolesova OE. Key role of succinate dehydrogenase in insulin-induced inactivation of protein tyrosine phosphatases. Bull Exp Biol Med. 2002;133:568–70. doi: 10.1023/A:1020229724717. [DOI] [PubMed] [Google Scholar]
- Pomytkin IA, Kolesova OE. Effect of insulin on the rate of hydrogen peroxide generation in mitochondria. Bull Exp Biol Med. 2003;135:541–2. doi: 10.1023/A:1025412915297. [DOI] [PubMed] [Google Scholar]
- Hwang J-J, Hur KC. Insulin cannot activate extracellular-signal-related kinase due to inability to generate reactive oxygen species in SK-N-BE(2) human neuroblastoma cells. Mol Cells. 2005;20:280–287. [PubMed] [Google Scholar]
- Rosen OM, Herrera R, Olowe Y, Petruzzelli LM, Cobb MH. Phosphorylation activates the insulin receptor tyrosine protein kinase. Proc Natl Acad Sci USA. 1983;80:3237–40. doi: 10.1073/pnas.80.11.3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tornqvist HE, Pierce MW, Frackelton AR, Nemenoff RA, Avruch J. Identification of insulin receptor tyrosine residues autophosphorylated in vitro. J Biol Chem. 1987;262:10212–9. [PubMed] [Google Scholar]
- White MF, Shoelson SE, Keutmann H, Kahn CR. A cascade of tyrosine autophosphorylation in the β-subunit activates the phosphotransferase of the insulin receptor. J Biol Chem. 1988;263:2969–2980. [PubMed] [Google Scholar]
- Zhang B, Tavare JM, Ellis L, Roth RA. The regulatory role of known tyrosine autophosphorylation sites of the insulin receptor kinase domain. An assessment by replacement with neutral and negatively charged amino acids. J Biol Chem. 1991;266:990–996. [PubMed] [Google Scholar]
- Tornqvist HE, Gunsalus JR, Nemenoff RA, Frackelton AR, Pierce MW, Avruch J. Identification of the insulin receptor tyrosine residues undergoing insulin-stimulated phosphorylation in intact rat hepatoma cells. J Biol Chem. 1988;263:350–359. [PubMed] [Google Scholar]
- Issad T, Tavare JM, Denton RM. Analysis of insulin receptor phosphorylation sites in intact rat liver cells by two-dimensional phosphopeptide mapping. Predominance of the trisphosphorylated form of the kinase domain after stimulation by insulin. Biochem J. 1991;275:15–21. doi: 10.1042/bj2750015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard SR, Wei L, Ellis L, Hendrickson WA. Crystal structure of the tyrosine kinase domain of the human insulin receptor. Nature. 1994;372:746–54. doi: 10.1038/372746a0. [DOI] [PubMed] [Google Scholar]
- Hubbard SR. Crystal structure of the activated insulin receptor tyrosine kinase in complex with peptide substrate and ATP analog. EMBO J. 1997;16:5572–81. doi: 10.1093/emboj/16.18.5572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wie L, Hubbard SR, Hendrickson WA, Ellis L. Expression, Characterization, and Crystallization of the Catalytic Core of the Human Insulin Receptor Protein-tyrosine Kinase Domain. J Biol Chem. 1995;270:8122–8130. doi: 10.1074/jbc.270.14.8122. [DOI] [PubMed] [Google Scholar]
- Boveris A, Oshino N, Chance B. The cellular production of hydrogen peroxide. Biochem J. 1972;128:617–30. doi: 10.1042/bj1280617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Votyakova TV, Reynolds IJ. DeltaPsi(m)-Dependent and – independent production of reactive oxygen species by rat brain mitochondria. J Neurochem. 2001;79:266–77. doi: 10.1046/j.1471-4159.2001.00548.x. [DOI] [PubMed] [Google Scholar]
- Loschen G, Azzi A, Richter C, Flohe L. Superoxide radicals as precursors of mitochondrial hydrogen peroxide. FEBS Lett. 1974;42:68–72. doi: 10.1016/0014-5793(74)80281-4. [DOI] [PubMed] [Google Scholar]
- Korshunov SS, Skulachev VP, Starkov AA. High protonic potential actuates a mechanism of production of reactive oxygen species in mitochondria. FEBS Lett. 1997;416:15–8. doi: 10.1016/S0014-5793(97)01159-9. [DOI] [PubMed] [Google Scholar]
- Korshunov SS, Korkina OV, Ruuge EK, Skulachev VP, Starkov AA. Fatty acids as natural uncouplers preventing generation of O2.- and H2O2 by mitochondria in the resting state. FEBS Lett. 1998;435:215–8. doi: 10.1016/S0014-5793(98)01073-4. [DOI] [PubMed] [Google Scholar]
- Andreyev AY, Kushnareva YE, Starkov AA. Mitochondrial metabolism of reactive oxygen species. Biochemistry (Mosc) 2005;70:200–14. doi: 10.1007/s10541-005-0102-7. [DOI] [PubMed] [Google Scholar]
- Bessman SP, Mohan C, Zaidise I. Intracellular site of insulin action: mitochondrial Krebs cycle. Proc Natl Acad Sci USA. 1986;83:5067–70. doi: 10.1073/pnas.83.14.5067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessman SP, Mohan C. Insulin as a probe of mitochondrial metabolism in situ. Mol Cell Biochem. 1997;174:91–6. doi: 10.1023/A:1006834408181. [DOI] [PubMed] [Google Scholar]
- Craft S. Insulin resistance syndrome and Alzheimer's disease: age- and obesity-related effects on memory, amyloid, and inflammation. Neurobiol Aging. 2005;26:65–9. doi: 10.1016/j.neurobiolaging.2005.08.021. [DOI] [PubMed] [Google Scholar]
- Hoyer S. Glucose metabolism and insulin receptor signal transduction in Alzheimer disease. Eur J Pharmacol. 2004;490:115–25. doi: 10.1016/j.ejphar.2004.02.049. [DOI] [PubMed] [Google Scholar]
- de la Monte SM, Wands JR. Review of insulin and insulin-like growth factor expression, signaling, and malfunction in the central nervous system: Relevance to Alzheimer's disease. J Alzheimers Dis. 2005;7:45–61. doi: 10.3233/jad-2005-7106. [DOI] [PubMed] [Google Scholar]
- Steen E, Terry BM, Rivera EJ, Cannon JL, Neely TR, Tavares R, Xu XJ, Wands JR, de la Monte SM. Impaired insulin and insulin-like growth factor expression and signaling mechanisms in Alzheimer's disease – is this type 3 diabetes? J Alzheimers Dis. 2005;7:63–80. doi: 10.3233/jad-2005-7107. [DOI] [PubMed] [Google Scholar]
- Andreeva N, Khodorov B, Stelmashook E, Cragoe E, Jr, Victorov I. Inhibition of Na+/Ca2+ exchange enhances delayed neuronal death elicited by glutamate in cerebellar granule cell cultures. Brain Res. 1991;548:322–5. doi: 10.1016/0006-8993(91)91141-M. [DOI] [PubMed] [Google Scholar]
- Kiedrowski L, Brooker G, Costa E, Wroblewski JT. Glutamate impairs neuronal calcium extrusion while reducing sodium gradient. Neuron. 1994;12:295–300. doi: 10.1016/0896-6273(94)90272-0. [DOI] [PubMed] [Google Scholar]
- Starkov AA, Fiskum G, Chinopoulos C, Lorenzo BJ, Browne SE, Patel MS, Beal MF. Mitochondrial alpha-ketoglutarate dehydrogenase complex generates reactive oxygen species. J Neurosci. 2004;24:7779–88. doi: 10.1523/JNEUROSCI.1899-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]