Abstract
We report an event-related potential (ERP) experiment of human recognition memory that explored the relation between conscious awareness and electrophysiological activity of the brain. We recorded ERPs from healthy adults while they made “remember” and “know” recognition judgments about previously seen words. These two kinds of judgments reflect “autonoetic” and “noetic” awareness, respectively. The ERP effects differed between the two kinds of awareness while they were similar for “true” and “false” recognition. Noetic awareness was associated with a temporoparietal positivity in the N400 range (325–600 ms) and a late (600–1,000 ms) frontocentral negativity, whereas autonoetic awareness was associated with a widespread, late, bifrontal and left parietotemporal (600–1000 ms) positivity. In the very late (1,300–1,900 ms) time window, a right frontal positivity was observed for both remember and know judgments of both true and false targets. These results provide physiological evidence for two types of conscious awareness in episodic memory retrieval.
Keywords: autonoetic and noetic awareness, brain physiology, remember, know, false recognition
One of the most daunting problems of cognitive neuroscience has to do with the identification of neural correlates of conscious awareness that people have of themselves and the world in which they live (1–4). Although a great deal is known and written about the brain anatomy, physiology, and chemistry of perception, attention, memory, and other categories of cognition, most of the available literature does not address the issue of conscious awareness. A long-standing difficulty of studying neural correlates of conscious awareness—the lack of techniques allowing in vivo measurement of brain activity—has now been largely overcome. Techniques such as electromagnetic recording and functional neuroimaging (5, 6) have made the future prospects in this respect appear brighter today than they have ever been before.
A second obstacle to the study of neural correlates of consciousness lies in the difficulty of teasing apart, at the cognitive level of analysis, processes that vary in their contribution to conscious awareness. Under normal conditions, these processes are inextricably bound together. Therefore, even if we could identify some neural events that are systematically related to a cognitive act, we would not know which aspects of these neural events reflect what component processes, or combinations of processes, of the act.
The difficulty caused by the tight coupling of conscious and nonconscious cognitive processes is well known to students of consciousness (7–14). The solution, too, is known in principle; if we wish to isolate neural correlates of conscious awareness, it is necessary to pry apart the conscious and nonconscious processes in cognition. Crick and Koch (8) have discussed possible approaches to this general problem, and Koch and Braun (15) have reported on the progress in the developments toward identifying the neuronal correlate of visual awareness.
Here we report an event-related potential (ERP) study of conscious awareness in memory for past events. We adopted an experimental design that allowed us to do two things: (i) differentiate between two different states of awareness of past events—“autonoetic” and “noetic” awareness (1, 16, 17) and (ii) differentiate the electrophysiological signatures of these two states of awareness from the electrophysiological signatures of the brain processes that did not contribute to these states. The logic of this design made it possible to relate subjective awareness to objective physiological measures of brain activity.
Background.
According to one theory of human memory there exist two different modes of access to, and two corresponding forms of conscious awareness of, information about previously experienced events: autonoetic and noetic awareness (1, 17). Autonoetic awareness (remembering) represents the standard experiential mode of the episodic memory system (1, 17). It is the kind of awareness that characterizes mental “re-living” of happenings from one’s personal past. It is phenomenally known to all healthy people who can “travel back in time” in their own minds. Noetic awareness (knowing) accompanies an individual’s interaction with its environment in the present. It is the standard experiential mode of retrieval operations in the semantic memory (general knowledge) system (18). When an individual thinks about the world, relying on her semantic memory, she is consciously aware of the relation between her thoughts and aspects of the world that are not perceptually present at the time. The experiential flavor of the noetic awareness is different from autonoetic awareness of personally experienced past events.
The remember/know (R/K) paradigm.
Autonoetic and noetic awareness of memory retrieval are operationally defined in terms of the R/K paradigm (1, 19–21). In the first phase of the procedure, subjects are exposed to a series of “miniature events”: discrete visual or auditory items appearing one at a time for a few seconds each. In the second phase, subjects are presented with both old (previously encountered) and new (previously not encountered) items, and asked to assign each such test item to one of three mutually exclusive experiential categories: (i) they remember the event of the item’s presentation in the study list (autonoetic awareness of the past); (ii) they know that the item was a part of the list, but they cannot actually recollect the event of its occurrence (noetic awareness of the past); or (iii) they have no awareness of any kind that the item was present in the earlier list (unawareness of the past).
The R/K paradigm is conceptually related to dual-process models of recognition memory (22–25), differing from these models primarily in its focus on subjectively experienced awareness rather than on hypothetical cognitive processes.
ERPs.
ERPs have been used extensively to investigate neural correlates of many cognitive processes, including memory retrieval. In standard recognition memory paradigms, ERPs elicited by repeated words are compared with ERPs elicited by words presented for the first time (26). The difference between them is thought to reflect processes of memory retrieval. The observed difference is usually referred to as the ERP repetition effect; it is typically manifested in more positive ERPs elicited by the repeated words (26). The ERP repetition effect extends over a broad time period starting around 200 ms (27, 28) and persisting until more than 1,500 ms after the presentation of the test word (29). This extended time course suggests that the ERP repetition effect is likely to comprise several aspects of memory retrieval, including conscious and unconscious processes, and, therefore, at least in principle, the ERP repetition effect could be used to identify neural correlates of conscious awareness of retrieval. However, as with other techniques, the major obstacle to mapping components of the ERP repetition effect to conscious awareness in memory retrieval lies in the difficulty of experimentally isolating processes carrying different forms of awareness. To circumvent this difficulty, we compared ERPs elicited during true and false recognition.
True and false recognition.
It has been known for some time that subjects in recognition memory tests are likely to make false positive responses to newly presented test items that are similar to studied items. The probability of occurrence of such false recognition can be experimentally manipulated. For verbal materials, an especially powerful paradigm for enhancing false recognition was initially constructed by Deese (30) and subsequently elaborated and further developed by Roediger and McDermott (31).
In this paradigm, subjects make R/K recognition judgments on three types of test words: words that were previously presented in a study list (true targets), words that were not previously presented but semantically (associatively) related to the presented words (false targets), and previously nonpresented and semantically unrelated distractor words (“new” words). A reliable finding is that perfectly normal and intelligent people frequently claim not only that they know that false targets were in the study list but also that they actually remember the events of the false targets’ appearance (31, 32). Thus, retrieval processing of both true and false targets can produce similar states of autonoetic and noetic awareness despite considerable differences in their preretrieval processing histories.
The logic of the experiment.
We combined the R/K procedure, true and false recognition manipulation, and the ERP technique for the purpose of identifying electrophysiological correlates of autonoetic and noetic awareness of retrieval. The logic of the experiment is schematically presented in Fig. 1. Exemplified for autonoetic but equally valid for noetic awareness, the logic was as follows. We assumed that the subject’s autonoetic awareness associated with R judgments is the same for true and false targets. We further assumed that the brain activity correlated with autonoetic awareness at retrieval is essentially the same regardless of whether it is elicited by a true target or a false target. Finally, we assumed that those components of the brain activity that are different for R and K judgments but indistinguishable for true and false targets reflect conscious awareness independent of nonconscious processes. Given these assumptions, the ERPs of R and K judgments that are similar for true and false targets reflect conscious awareness of memory retrieval.
Figure 1.
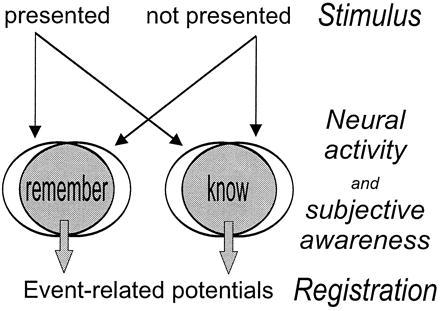
Our conceptual approach. Stimuli that were previously presented (true targets) and not presented (false targets) evoke similar subjective awareness of retrieval, and similar correlated changes in the brain’s electrophysiological activity as measured by ERPs. Thus, the ERPs of R and K judgments that are similar for true and false targets reflect conscious awareness of memory retrieval.
METHODS
Sixteen right-handed subjects (seven males) were visually presented with a study list and a test list. The study list was composed of 24 blocked sets of 12 semantically related words. These were taken from 24 sets of 15 semantic associates of a category word (e.g., sleep: tired, awake, dream, bed, wake, snooze, blanket, doze, slumber, snore, nap, peace, yawn, drowsy, and rest). The category word (sleep) together with the highest three semantic associates (e.g., tired, awake, and dream) were not presented in the study phase but served as false targets in the test list. During the study phase, words appeared at a rate of one word every 1.7 s (1,200 ms presentation and 500 ms fixation point), and subjects were instructed to study the words for a later memory test. After the study phase, there was a 30-min delay period during which time subjects were fitted with an electrode cap. Subjects then received a recognition memory test that contained 96 true targets, 96 false targets, and 96 new distractor words. The three types of words were randomly ordered and balanced for word length and frequency of occurrence in the language.
Each test trial began with the presentation of a fixation cross for 500 ms, followed by a test word for 300 ms. The word was then replaced by a fixation cross for 1,700 ms, followed by an R/K prompt which remained on the screen for 3,000 ms. Subjects were instructed to decide for each word whether it was presented in the study list (old/new response) and whether they remembered or knew (R/K judgment) that it was presented. They were required to respond R if they could remember anything about the actual event of the word’s occurrence in the study list, re-experiencing the episode, and to respond K if they knew that the word had been a part of the list but could not remember anything about its occurrence. Subjects were instructed to make their recognition judgments as accurately and quickly as they could. They were told to wait until after the R/K prompt appeared on the screen before making their R/K response, but to make it within 3 s of the presentation of the prompt. Responses were made with the index and middle fingers, and the response hand was counterbalanced across subjects. The old/new responses were timed; the R/K judgments were not. For items receiving an initial new response, subjects were required to press the R/K response keys simultaneously.
The ERPs were recorded with 29 tin electrodes, mounted in an electrocap (Electro-Cap International, Eaton, OH) according to the International 10–20 System (F7, F3, Fz, Cz, Pz, P3, T7, T5, O1, IN1, INZ, Fc1, C3p, Cp1, Po1, To1, and corresponding right hemisphere sites). All electrodes were referred to the left mastoid. Additional electrodes located at the outer canthi of both eyes and below the right eye were used to monitor eye blinks and movements. The electroencephalogram signals were amplified with a band pass of 0.01–100 Hz and digitized at a rate of 250 Hz. Artifacts due to blinks, saccades, excessive muscle activity and amplifier saturation were rejected off-line. The electroencephalogram signals were averaged time locked to the onset of each word presentation over epochs of 2,100 ms, with a presampling period of 200 ms. Thus, the averaged ERP wave forms encompass the entire 2-s time period between the onset of the presentation of each test word and the appearance of the R/K prompt.
Electrophysiological results were analyzed by comparing ERP wave forms elicited by correctly rejected distractor words, and the words representing the four experimental outcomes of interest: remembered true targets, known true targets, remembered false targets, and known false targets. Statistical analyses were performed with mean voltage amplitudes of ERP wave forms in designated time windows, using ANOVA that included Greenhouse–Geisser corrections in cases where factors had more than two levels. The analyses were conducted on all 29 electrode sites unless stated otherwise. All reported differences are significant at least at a level of P < 0.05.
RESULTS
The behavioral data, summarized in Table 1, were in line with previous results obtained with the false recognition paradigm; subjects made numerous R and K judgments of both true and false targets. Subjects produced sizable proportions of all four kinds of judgments of interest. The mean latency of the old responses preceding the R/K decision was approximately 1,100 ms. There was no significant latency difference between the old responses preceding R/K judgments.
Table 1.
Behavioral results
| Response | True targets | False targets | Distractors |
|---|---|---|---|
| Remember | 0.38 | 0.22 | 0.06 |
| Know | 0.25 | 0.28 | 0.15 |
| Total old responses | 0.63 | 0.50 | 0.21 |
Behavioral results of R and K judgments show prominent false recognition. Proportions of R/K judgments to true and false targets are significantly different from distractors (Student’s t test P values < 0.001 in all comparisons).
Fig. 2 shows the grand-average ERP wave forms of the entire recording period (0–1,900 ms) elicited by correctly rejected distractor words (henceforth called distractors), R and K judgments of true targets, and R and K judgments of false targets. In the following analysis, we will use the term “ERP recognition effect” to refer to the difference between the ERPs elicited by recognized target words and ERPs elicited by distractors.
Figure 2.
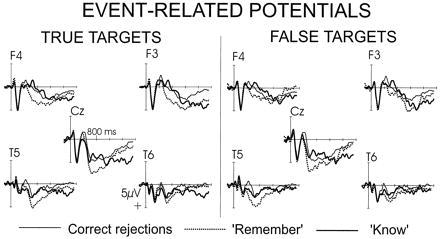
Grand-average ERP wave forms elicited R and K judgments to true and false targets, as well as correct rejections of distractors. The wave forms are displayed over the entire recording period of 1,900 ms after the onset of the test word. Significant differences between the three response categories were observed in four time windows, 190–200 ms, 300–600 ms, 600–1,000 ms, and 1,300–1,900 ms. Subjects made old/new discriminations at approximately 1,100 ms. The R/K judgment was prompted at the end of the recording period.
Significant ERP recognition effects were elicited in four time windows, 190–200 ms, 300–600 ms, 600–1,000 ms, and 1,300–1,900 ms. Results will be reported separately for each of these time periods.
Time Window 190–200 ms.
In this time window, the ERP recognition effect was a positivity confined to posterior electrodes (T5 and T6) and elicited only by K judgments to true targets [F(1, 15) = 6.60, P < 0.02, laterality, P < 0.12].
Time Window 300–600 ms.
In this time window, ERP recognition effects were also confined mainly to posterior electrodes and will be reported for electrodes T5 and T6. The ERP recognition effects for K judgments were indistinguishable for true and false recognition and appeared as a positivity over the entire 300- to 600-ms time period [true recognition (rec.): F(1, 15) = 8.88, P < 0.01; false rec.: F(1, 15) = 16.04, P < 0.001]. This positivity was significantly greater over the left than the right side for K judgments to true targets [F(1, 15) = 6.22, P < 0.025] but not for false targets (P < 0.5). The ERP recognition effect for R judgments was different for true and false recognition; remembered true targets elicited a positivity with an onset at 450 ms, lateralized to the left hemisphere [F(1, 15) = 8.95, P < 0.01, laterality, F(1, 15) = 9.56, P < 0.01]. Remembered false targets elicited a bilateral positivity by 325 ms [F(1, 15) = 38.62, P < 0.0001, laterality, P < 0.12]. A direct comparison of R and K judgments showed different tendencies for true and false targets; whereas ERPs to R judgments were bilaterally more positive than K judgments for false targets [F(1, 15) = 5.03, P < 0.05, laterality, P < 0.25], there was a tendency toward bilateral negativity for true targets in the time window 350–400 ms [F(1, 15) = 3.54, P < 0.08, laterality, P < 0.50].
Time Window 600–1,000 ms.
In this time window, the ERP recognition effects were more widespread and consistent across true and false targets. The ERP recognition effect for R judgments was apparent in a widespread positivity [true rec.: F(1, 15) = 11.12, P < 0.01; false rec.: F(1, 15) = 7.81, P < 0.025]. This positivity appeared to be significantly lateralized to the left hemisphere over temporoparietal electrodes (T5 and T6; P < 0.01) but not over frontal electrodes (F3 and F4; P < 0.5). The ERP recognition effect for K judgments was apparent in a negativity that appeared to be most prominent over frontocentral regions without being lateralized [true rec.: F(1, 15) = 4.49, P < 0.05; false rec.: F(1, 15) = 6.51, P < 0.025]. The direct comparison of R and K judgments showed significantly greater positivity for R judgments [true rec.: F(1, 15) = 12.51, P < 0.01; false rec.: F(1, 15) = 14.29, P < 0.01].
Fig. 3 graphically depicts the similarity of the ERP recognition effects across true and false targets in the 600- to 1,000-ms time window. The figure shows scalp topographic voltage maps of the ERP recognition effect based on R and K judgments. The voltage values are derived from mean amplitudes of corresponding ERPs after subtraction of ERPs elicited by distractors. Voltage maps of ERPs differed for R and K judgments, but they were similar for true and false targets.
Figure 3.
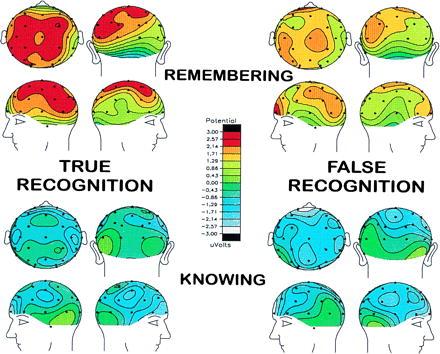
Scalp topographic voltage maps of ERPs elicited by R (upper maps) and K (lower maps) responses. True recognition is displayed on the left, and false recognition is displayed on the right. Voltage values are derived from mean amplitudes of grand-average ERPs after subtraction of ERPs elicited by correct rejections of distractors. The depicted time interval is 600–1,000 ms after the onset of each test word. The figure shows that the voltage maps differ for R and K responses but are remarkably similar for true and false recognition.
Time Window 1,300–1,900 ms.
In this time window, ERP recognition effects were confined to right frontal and frontocentral electrodes and were indistinguishable for true and false recognition. The ERP recognition effect for both R [laterality (F3 and F4); true rec.: F(1, 15) = 7.62, P < 0.025; false rec.: F(1, 15) = 13.19, P < 0.01] and K [true rec.: F(1, 15) = 4.86, P < 0.05; false rec.: F(1, 15) = 10.05, P < 0.01] judgments appeared in the form of a circumscribed right frontal positivity, although the positivity was spread more widely for K than R judgments [judgment type (R vs. K) by electrode (Cz vs. F4) interaction; F(1, 15) = 6.08, P < 0.025].
The ERP differences between true and false targets were examined in more detail by subtracting the ERPs associated with false targets from those associated with true targets. Fig. 4 presents the difference waves for the T5 and T6 electrodes in the 0- to 800-ms window. The absence of any differences between true and false targets would result in a flat difference wave. Differences were restricted to temporoparietal electrode sites and the time windows 190–200 ms and 300–600 ms. In the window 190–200 ms, ERPs to true K judgments were more positive than false K judgments [F(1, 15) = 7.57, P < 0.025], mainly over the left hemisphere [F(1, 15) = 6.79, P < 0.025]. No such differences were present for the R judgments (P < 0.60, laterality, P < 0.60). In the time window 300–600 ms, false R judgments elicited more positive ERPs than true R judgments [F(1, 15) = 8.64, P < 0.01] bilaterally (P < 0.6). No such differences were present for the K judgments (P < 0.95, laterality, P < 0.25).
Figure 4.
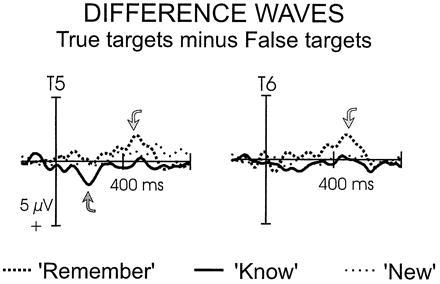
ERP difference waves depicting the difference between true and false recognition. Wave forms are plotted from 0–800 ms for left and right temporoparietal electrodes (T5 and T6). Significant differences appear between 190–200 ms and 300–600 ms.
DISCUSSION
The aim of the present investigation was to identify the neural correlates of autonoetic and noetic awareness in memory retrieval. To that end, we compared electrophysiological (ERP) measures associated with R and K judgments across true and false targets.
Our behavioral data confirmed what was already known; subjects made R and K judgments of both of true and false targets, implying that they could not distinguish true targets from false. This occurred despite the fact that true and false targets varied considerably from each other with respect to their prior intraexperimental sensory, perceptual, and lexical processing history. Such a history was present for true targets and absent for false targets. Thus, in many cases, these preretrieval processing differences did not affect the subjects’ conscious awareness at retrieval.
The novel finding was that the two distinctive states of awareness reflected in the subjective reports of test words were also distinctive electrophysiologically. When compared with ERPs correlated with the absence of conscious awareness of any earlier occurrence of given words (correct rejections of distractors), autonoetic awareness of such events (R judgments) was associated with a late, widespread, bifrontal and left temporoparietal positivity in the time window 600–1,000 ms. In a similar comparison, noetic awareness (K judgments) was associated with a frontocentral negativity in the same time window, preceded by a bilateral temporoparietal positivity in the time window 300–600 ms.
The temporal and spatial characteristics of the effects seen in the time window 300–600 ms are reminiscent of the modulation of what is known as the N400 component in studies of word repetition and semantic priming (27, 33, 34). The major difference lies in the longevity of the effect (at least 30 min) in our experiment, which exceeds previously established limits (35). Our findings help to further understand the nature of previously established links between the N400 effect and episodic (36) and controlled (34) memory processes. Because our false targets showed the same N400 modulation as true targets, the data suggest that the N400 recognition “repetition” effect may represent conscious awareness in memory retrieval, but it is mainly true for one type of awareness, noetic awareness.
In the late time window, ERPs related to noetic awareness were more negative not only in comparison with ERPs related to autonoetic awareness but also in comparison to ERPs elicited by distractors. A similar relative negativity for repeated words has been reported previously (37), where the repetition was embedded within a continuous text. Reading a continuous text may evoke the same kind of noetic awareness of word repetition that was observed in the current experiment for knowing judgments.
Autonoetic awareness was correlated with a late positivity whose temporal and spatial characteristics agree well with the late positive component (LPC) that has often been observed in ERP studies of recognition (26, 38). The LPC has been shown to be elicited by words that are repeated and recognized (29, 39, 40). It has been a matter of considerable debate as to what extent the LPC is actually related to recognition accompanied by conscious recollection as opposed to familiarity (41–43). Our results contribute to this issue in two respects. First, they show that the LPC was associated exclusively with R judgments; it was not observed for K judgments. Second, because perceptual priming was probably present for true targets, but not for false targets, the LPC was elicited by R judgments independently of “automatic” or nonconscious processes that underlie such priming. Thus, the LPC seems to be related to autonoetically conscious recollection.
In previous studies, such conscious recollection has been identified with the ability to make correct source judgments (29, 44). These studies observed a marked LPC only when subjects could correctly identify the source of a recognized word. In our experiment, false recognition (R and K judgments of false targets) is comparable to making false source judgments. If so, our data show that subjects can have a recollective experience even when they make incorrect source judgments. Thus, because the LPCs associated with R judgments were indistinguishable for true and false targets, it looks as if LPC is not correlated with source judgments as such. Rather, it reflects the presence of autonoetic awareness that accompanies retrieval of source information.
In the very late time window, 1,300–1,900 ms, both R and K judgments elicited a prominent right frontal positivity relative to correctly rejected distractors. This positivity seemed to spread more widely for K judgments. Right frontal effects have been previously observed in both ERP studies (29, 44) and positron-emission tomography studies of memory (45). Two alternative hypotheses, not necessarily exclusive, have been proposed. One holds that right prefrontal regions “set the stage” (46) for episodic memory retrieval by establishing a general episodic retrieval mode (47–49). The other hypothesis is that the activity of right frontal lobe regions signals item-specific processes associated with the successful recognition of items as old (50, 51).
Our data are neutral with respect to the first hypothesis because the subjects in our experiment were always in the episodic retrieval mode. But the data agree with the second hypothesis. The ERP differences between R and K judgments indicate that right frontal effects were item-specific. The ERP difference between R and K judgments furthermore suggests that the item-specific aspects of the right frontal effects may depend on the nature of awareness accompanying retrieval. This idea is supported by the fact that the right frontal positivity was the same across true and false targets.
CONCLUSION
In conclusion, this experiment has provided physiological evidence for two types of conscious awareness in episodic memory retrieval, autonoetic and noetic. The results showed a direct relation between subjective R and K judgments associated with true and false recognition of test words, on the one hand, and distinct patterns of the brain’s electrophysiological activity as measured by ERPs on the other.
The critical feature of the results is that the coupling between states of awareness and ERPs was very similar for both true and false targets. This means that the ERPs, like the states of subjective awareness, were not sensitive to the differences in the pre-retrieval processing of the two kinds of targets (presented vs. not presented). Because they were sensitive to the subjects’ states of conscious awareness (remembering vs. knowing), we can infer that the ERPs directly reflected the underlying neural changes associated with consciousness. Conversely, the subjects’ subjective reports of their states of awareness represented a more-or-less direct “readout” of their brain states.
The observed parallel dissociation of the two forms of awareness at two levels of analysis, psychological and physiological, under conditions where ERPs could be directly related to the subjects’ states of conscious awareness of retrieval, suggests that the neural activity associated with autonoetic awareness is at least partly different from that associated with noetic awareness. Although the results of our experiment speak directly to the issue of neural correlates of two forms of conscious access to the experienced past, more broadly, they illustrate how the concepts and methods of cognitive neuroscience can be brought to bear on the relation between brain activity and conscious thought.
Acknowledgments
We thank Eva McGrath for help with the preparation of the manuscript. This report was supported by grants from the National Institute of Mental Health, the National Institute of Neurological Disorders and Stroke (to G.R.M.), the Natural Sciences and Engineering Council of Canada, and the Deutsche Forschungsgemeinschaft (Grants HE 1531/3–1 and Du 280/1–1 to H.-J.H. and E.D.).
ABBREVIATIONS
- ERP
event-related potential
- R/K
remember/know
- LPC
late positive component
- rec.
recognition
References
- 1.Tulving E. Can Psychol. 1985;26:1–12. [Google Scholar]
- 2.Crick F, Koch C. Nature (London) 1995;375:121–123. doi: 10.1038/375121a0. [DOI] [PubMed] [Google Scholar]
- 3.Markowitsch H J. Neuropsychologia. 1995;33:1181–1192. doi: 10.1016/0028-3932(95)00057-a. [DOI] [PubMed] [Google Scholar]
- 4.Delacour J. Neuropsychologia. 1995;33:1061–1074. doi: 10.1016/0028-3932(95)00048-8. [DOI] [PubMed] [Google Scholar]
- 5.Lounasmaa O V, Hamalainen M, Hari R, Salmelin R. Proc Natl Acad Sci USA. 1996;20:8809–8815. doi: 10.1073/pnas.93.17.8809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Posner M I, Raichle M E. Images of Mind. New York: Sci. Am. Books; 1994. [Google Scholar]
- 7.Baars B J. A Cognitive Theory of Consciousness. Cambridge, U.K.: Cambridge Univ. Press; 1988. [Google Scholar]
- 8.Crick F, Koch C. Sci Am. 1992;267(3):153–159. doi: 10.1038/scientificamerican0992-152. [DOI] [PubMed] [Google Scholar]
- 9.Kinsbourne M. In: The Cognitive Neurosciences. Gazzaniga M S, editor. Cambridge, MA: MIT Press; 1995. pp. 1321–1329. [Google Scholar]
- 10.Donald M. Neuropsychologia. 1995;33:1087–1102. doi: 10.1016/0028-3932(95)00050-d. [DOI] [PubMed] [Google Scholar]
- 11.Gazzaniga M S. The Social Brain: Discovering the Networks of the Brain. New York: Basic Books; 1985. [Google Scholar]
- 12.Moscovitch M. In: The Cognitive Neurosciences. Gazzaniga M S, editor. Cambridge, MA: MIT Press; 1995. pp. 1341–1356. [Google Scholar]
- 13.Schacter D L. In: Varieties of Memory and Consciousness: Essays in Honour of Endel Tulving. Roediger L III, Craik F I M, editors. Hillsdale, NJ: Erlbaum; 1989. pp. 355–389. [Google Scholar]
- 14.Shallice T. From Neuropsychology to Mental Structure. Cambridge, U.K.: Cambridge Univ. Press; 1988. [Google Scholar]
- 15.Koch C, Braun J. Curr Opin Neurobiol. 1996;6:158–164. doi: 10.1016/s0959-4388(96)80068-7. [DOI] [PubMed] [Google Scholar]
- 16.Perner J, Ruffman T. J Exp Child Psychol. 1995;59:516–548. doi: 10.1006/jecp.1995.1024. [DOI] [PubMed] [Google Scholar]
- 17.Wheeler, M. E., Stuss, D. T. & Tulving, E. (1997) Psychol. Bull., in press. [DOI] [PubMed]
- 18.Schacter D L, Tulving E. In: Memory Systems 1994. Schacter D L, Tulving E, editors. Cambridge, MA: MIT Press; 1994. pp. 1–38. [Google Scholar]
- 19.Gardiner J M. Mem Cognit. 1988;16:309–313. doi: 10.3758/bf03197041. [DOI] [PubMed] [Google Scholar]
- 20.Gardiner J M, Java R I, Richardson-Klavehn A. Can J Exp Psychol. 1996;50:114–122. [Google Scholar]
- 21.Gardiner J M, Java R I. In: Theories of Memory. Collins A, Conway M A, Gathercole S E, Morris P E, editors. Hillsdale, NJ: Erlbaum; 1993. pp. 163–188. [Google Scholar]
- 22.Atkinson R C, Juola J F. In: Contemporary Developments in Mathematical Psychology. Krantz D H, Atkinson R C, Luce R D, Suppes P, editors. Vol. 1. San Francisco: Freeman; 1974. pp. 243–293. [Google Scholar]
- 23.Jacoby L L. J Mem Lang. 1991;30:513–541. [Google Scholar]
- 24.Mandler G. Psychol Rev. 1980;87:252–271. [Google Scholar]
- 25.Yonelinas A P. J Exp Psychol Learn Mem Cognit. 1994;20:1341–1354. doi: 10.1037//0278-7393.20.6.1341. [DOI] [PubMed] [Google Scholar]
- 26.Rugg M D. In: Electrophysiology of the Mind. Rugg M D, Coles M G H, editors. Vol. 25. Oxford: Oxford Univ. Press; 1995. pp. 133–170. [Google Scholar]
- 27.Rugg M D. Psychophysiology. 1985;22:642–647. doi: 10.1111/j.1469-8986.1985.tb01661.x. [DOI] [PubMed] [Google Scholar]
- 28.Besson M, Kutas M, Van Patten C. J Cognit Neurosci. 1992;4:132–149. doi: 10.1162/jocn.1992.4.2.132. [DOI] [PubMed] [Google Scholar]
- 29.Wilding E, Rugg M D. Brain. 1996;119:889–905. doi: 10.1093/brain/119.3.889. [DOI] [PubMed] [Google Scholar]
- 30.Deese J. J Exp Psychol. 1959;58:17–22. doi: 10.1037/h0046671. [DOI] [PubMed] [Google Scholar]
- 31.Roediger H L, McDermott K B. J Exp Psychol Learn Mem Cognit. 1995;21:803–814. [Google Scholar]
- 32.McDermott K B. J Mem Lang. 1996;35:212–230. [Google Scholar]
- 33.Brown C, Hagoort P. J Cognit Neurosci. 1993;5:34–44. doi: 10.1162/jocn.1993.5.1.34. [DOI] [PubMed] [Google Scholar]
- 34.Bentin S, McCarthy G. J Exp Psychol Learn Mem Cognit. 1994;20:130–149. doi: 10.1037//0278-7393.20.3.595. [DOI] [PubMed] [Google Scholar]
- 35.Rugg M D, Nagy M E. Mem Cognit. 1989;15:473–481. doi: 10.3758/bf03198381. [DOI] [PubMed] [Google Scholar]
- 36.Besson M, Kutas M. J Exp Psychol Learn Mem Cognit. 1993;19:1115–1133. doi: 10.1037//0278-7393.19.5.1115. [DOI] [PubMed] [Google Scholar]
- 37.Van Petten C, Kutas M, Kluender R, Mitchiner M, McIsaac H. J Cognit Neurosci. 1991;3:131–150. doi: 10.1162/jocn.1991.3.2.131. [DOI] [PubMed] [Google Scholar]
- 38.Sanquist T F, Rohrbaugh J W, Syndulko K, Lindsley D B. Psychophysiology. 1980;17:568–576. doi: 10.1111/j.1469-8986.1980.tb02299.x. [DOI] [PubMed] [Google Scholar]
- 39.Paller K A, Kutas M. J Cognit Neurosci. 1992;4:375–391. doi: 10.1162/jocn.1992.4.4.375. [DOI] [PubMed] [Google Scholar]
- 40.Paller K A, Kutas M, McIsaac H K. Psychol Sci. 1995;6:107–111. [Google Scholar]
- 41.Rugg M D, Doyle M C. J Cognit Neurosci. 1992;4:69–79. doi: 10.1162/jocn.1992.4.1.69. [DOI] [PubMed] [Google Scholar]
- 42.Smith M, Halgren E. J Exp Psychol Learn Mem Cognit. 1989;15:50–60. doi: 10.1037//0278-7393.15.1.50. [DOI] [PubMed] [Google Scholar]
- 43.Smith M E. J Cognit Neurosci. 1993;5:1–13. doi: 10.1162/jocn.1993.5.1.1. [DOI] [PubMed] [Google Scholar]
- 44.Senkfor A, Van Petten C. Psychophysiology. 1995;32:F69. (abstr.). [Google Scholar]
- 45.Nyberg L, Cabeza R, Tulving E. Psychonom Bull Rev. 1996;3:134–147. doi: 10.3758/BF03212412. [DOI] [PubMed] [Google Scholar]
- 46.Tulving E, Markowitsch H J, Craik F I M, Habib R. Cereb Cortex. 1996;6:71–79. doi: 10.1093/cercor/6.1.71. [DOI] [PubMed] [Google Scholar]
- 47.Kapur S, Craik F I M, Jones C, Brown G M, Houle S, Tulving E. NeuroReport. 1995;6:1880–1884. doi: 10.1097/00001756-199510020-00014. [DOI] [PubMed] [Google Scholar]
- 48.Nyberg L, Tulving E, Habib R, Nilsson L-G, Kapur S, Houle S, Cabeza R, McIntosh A R. NeuroReport. 1995;7:249–252. [PubMed] [Google Scholar]
- 49.Schacter D L, Alpert N M, Savage C R, Rauch S L, Albert M S. Proc Natl Acad Sci USA. 1996;93:321–326. doi: 10.1073/pnas.93.1.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McIntosh, A. R., Nyberg, L., Bookstein, F. L. & Tulving, E. (1997) Hum. Brain Mapp., in press. [DOI] [PubMed]
- 51.Rugg M D, Fletcher P C, Frith C D, Frackowiak R S J, Dolan R J. Brain. 1996;119:2073–2083. doi: 10.1093/brain/119.6.2073. [DOI] [PubMed] [Google Scholar]


