Abstract
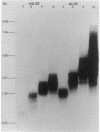
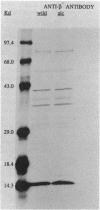
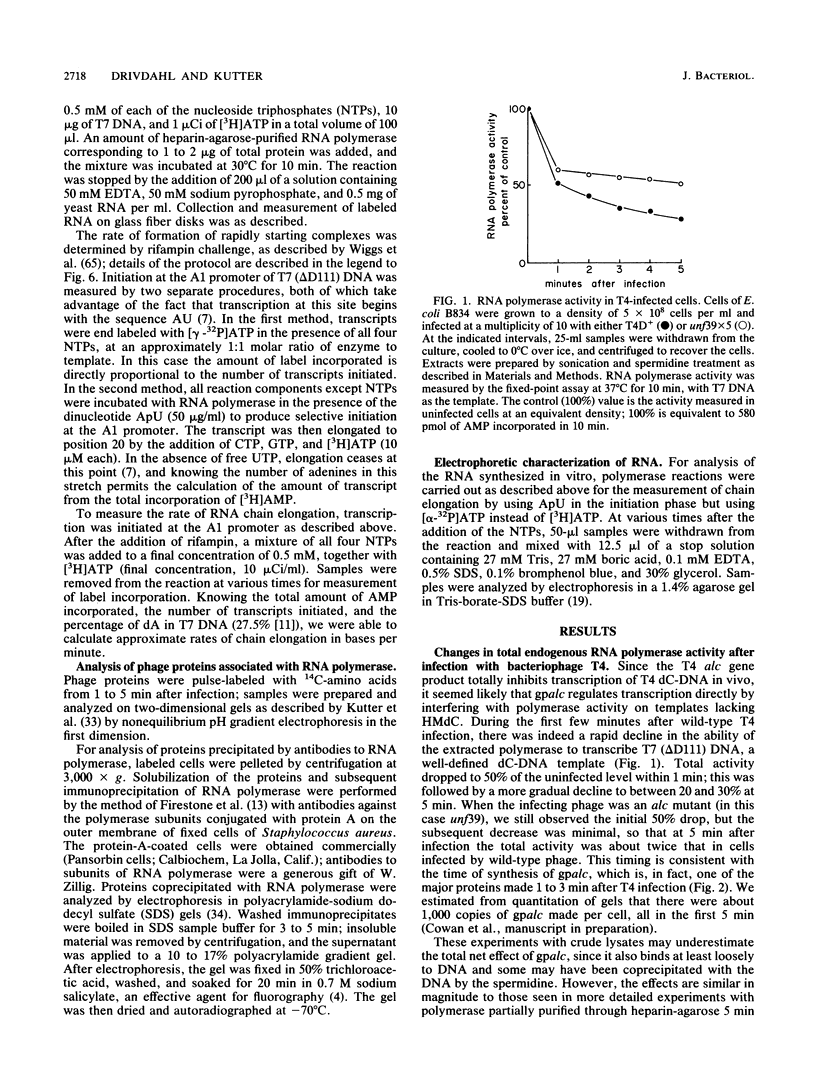
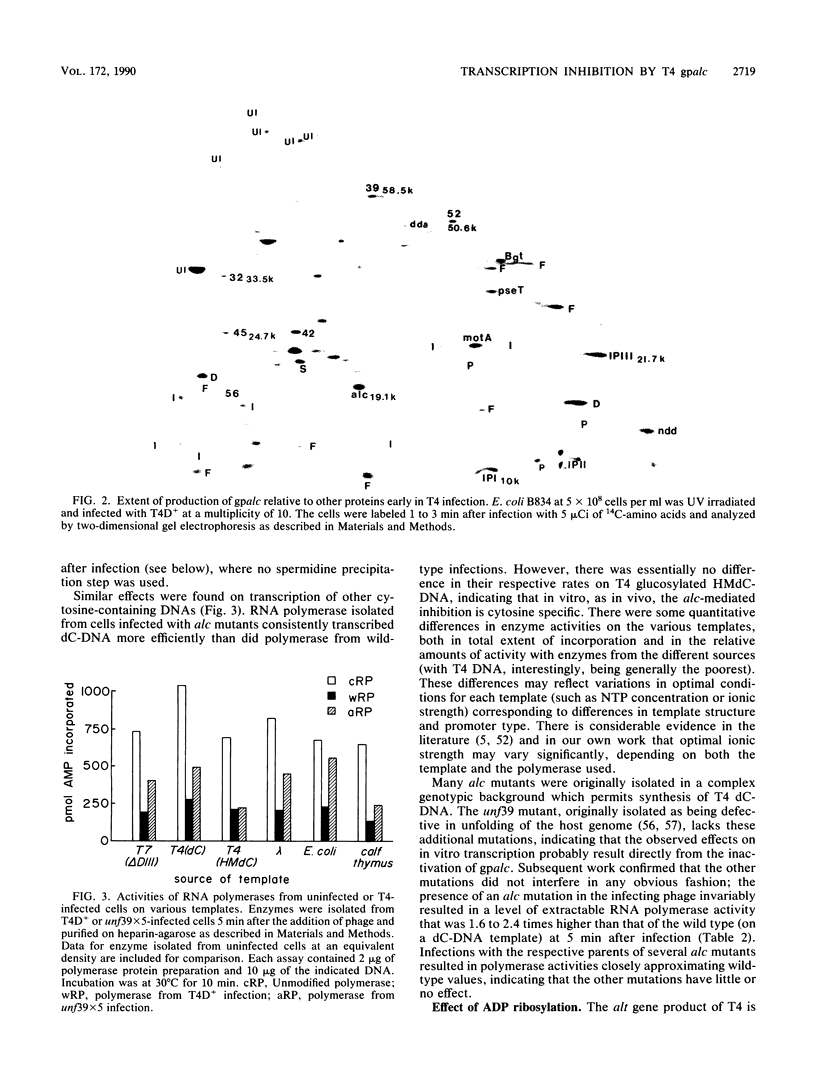
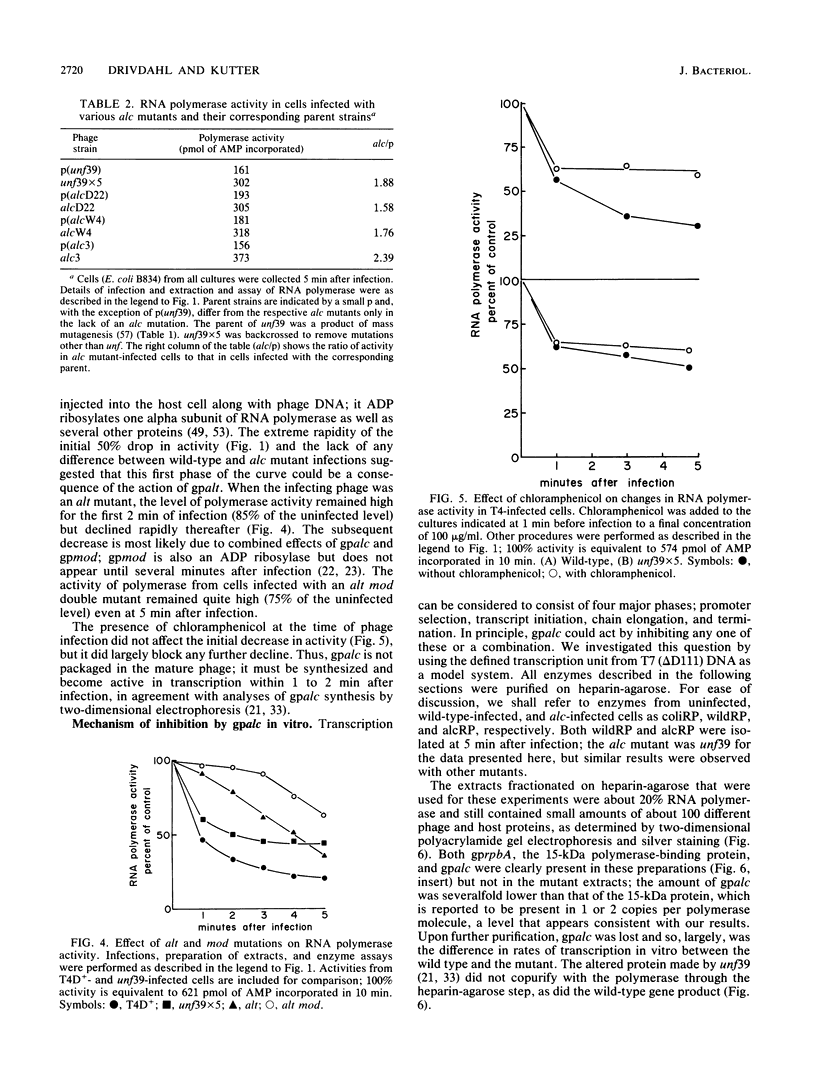
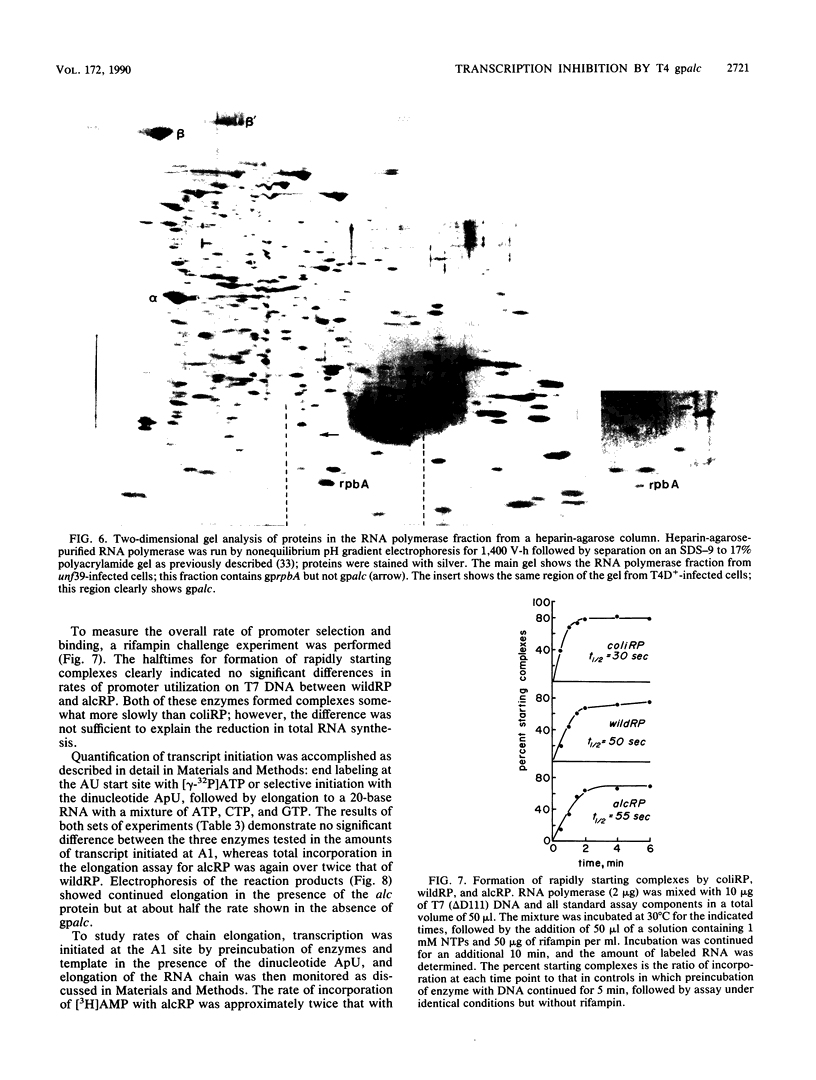
The alc gene product (gpalc) of bacteriophage T4 inhibits the transcription of cytosine-containing DNA in vivo. We examined its effect on transcription in vitro by comparing RNA polymerase isolated from Escherichia coli infected with either wild-type T4D+ or alc mutants. A 50 to 60% decline in RNA polymerase activity, measured on phage T7 DNA, was observed by 1 min after infection with either T4D+ or alc mutants; this did not occur when the infecting phage lacked gpalt. In the case of the T4D+ strain but not alc mutants, this was followed by a further decrease. By 5 min after infection the activity of alc mutants was 1.5 to 2.5 times greater than that of the wild type on various cytosine-containing DNA templates, whereas there was little or no difference in activity on T4 HMdC-DNA, in agreement with the in vivo specificity. Effects on transcript initiation and elongation were distinguished by using a T7 phage DNA template. Rifampin challenge, end-labeling with [gamma-32P]ATP, and selective initiation with a dinucleotide all indicate that the decreased in vitro activity of the wild-type polymerase relative to that of the alc mutants was due to inhibition of elongation, not to any difference in initiation rates. Wild-type (but not mutated) gpalc copurified with RNA polymerase on heparin agarose but not in subsequent steps. Immunoprecipitation of modified RNA polymerase also indicated that gpalc was not tightly bound to RNA polymerase intracellularly. It thus appears likely that gpalc inhibits transcript elongation on cytosine-containing DNA by interacting with actively transcribing core polymerase as a complex with the enzyme and cytosine-rich stretches of the template.
Full text
PDF
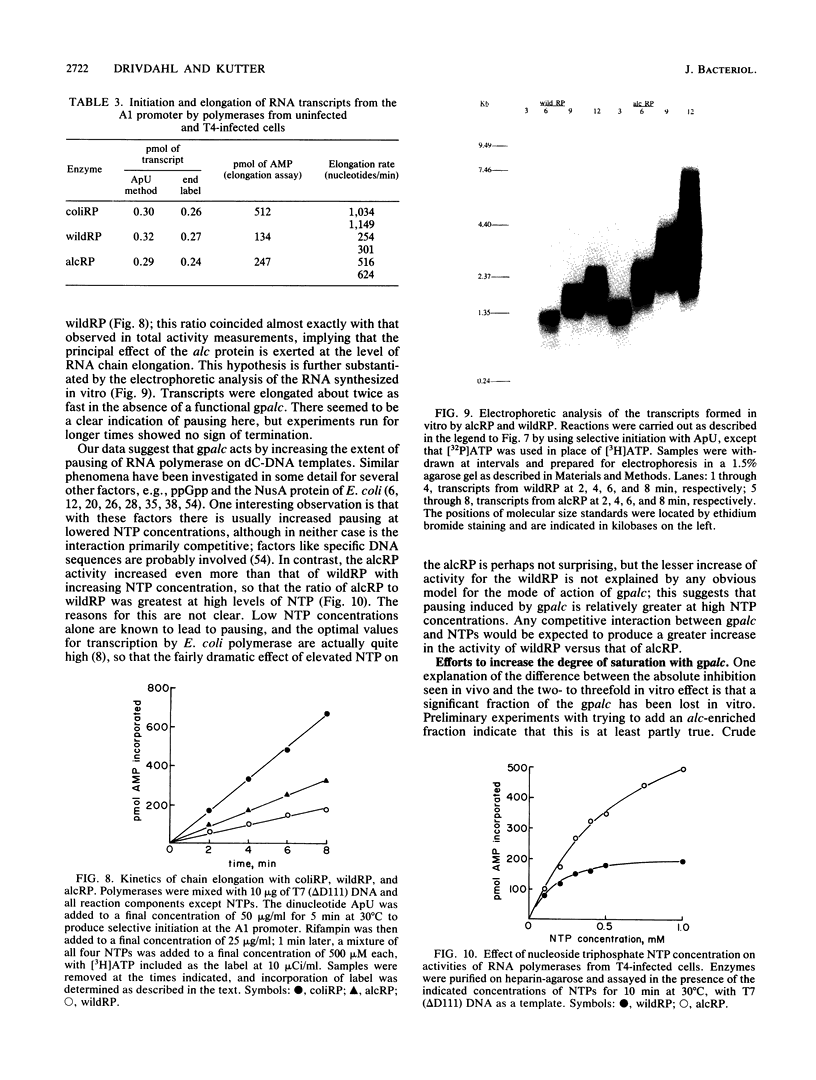
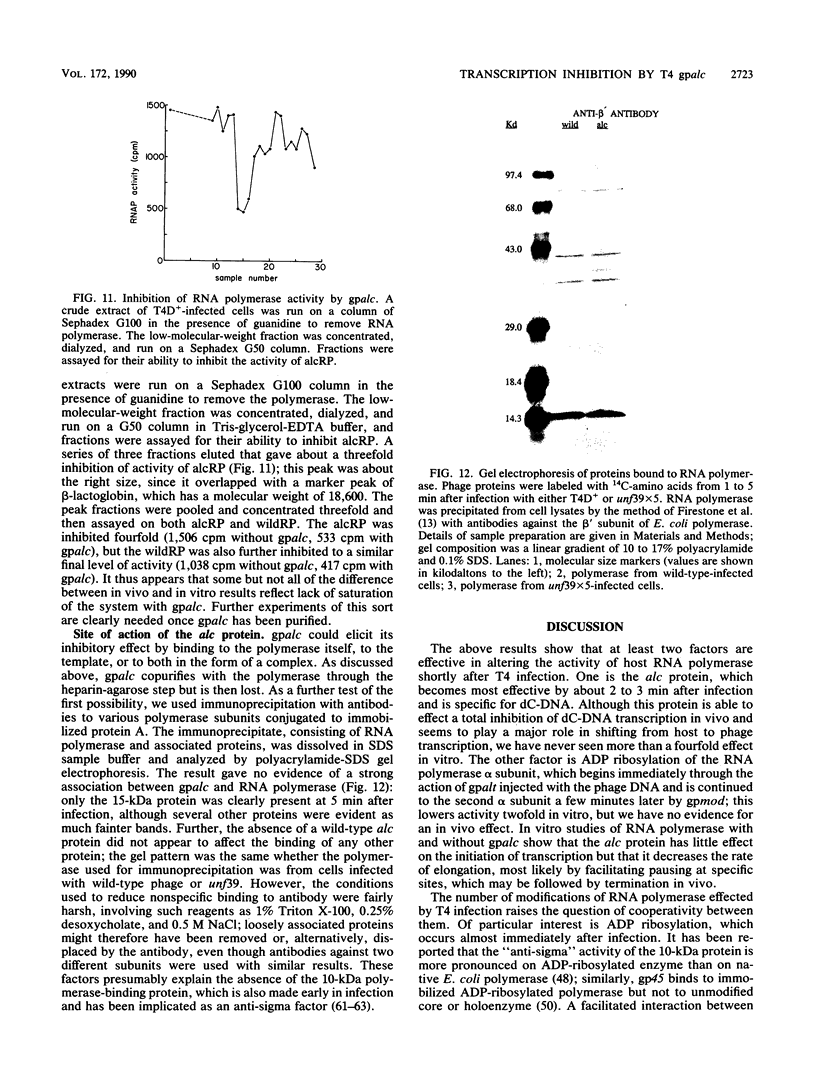




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burgess R. R., Jendrisak J. J. A procedure for the rapid, large-scall purification of Escherichia coli DNA-dependent RNA polymerase involving Polymin P precipitation and DNA-cellulose chromatography. Biochemistry. 1975 Oct 21;14(21):4634–4638. doi: 10.1021/bi00692a011. [DOI] [PubMed] [Google Scholar]
- Carlson K., Overvatn A. Bacteriophage T4 endonucleases II and IV, oppositely affected by dCMP hydroxymethylase activity, have different roles in the degradation and in the RNA polymerase-dependent replication of T4 cytosine-containing DNA. Genetics. 1986 Nov;114(3):669–685. doi: 10.1093/genetics/114.3.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain J. P. Fluorographic detection of radioactivity in polyacrylamide gels with the water-soluble fluor, sodium salicylate. Anal Biochem. 1979 Sep 15;98(1):132–135. doi: 10.1016/0003-2697(79)90716-4. [DOI] [PubMed] [Google Scholar]
- Chamberlin M. J., Nierman W. C., Wiggs J., Neff N. A quantitative assay for bacterial RNA polymerases. J Biol Chem. 1979 Oct 25;254(20):10061–10069. [PubMed] [Google Scholar]
- Chamberlin M., Kingston R., Gilman M., Wiggs J., deVera A. Isolation of bacterial and bacteriophage RNA polymerases and their use in synthesis of RNA in vitro. Methods Enzymol. 1983;101:540–568. doi: 10.1016/0076-6879(83)01037-x. [DOI] [PubMed] [Google Scholar]
- Davison B. L., Leighton T., Rabinowitz J. C. Purification of Bacillus subtilis RNA polymerase with heparin-agarose. In vitro transcription of phi 29 DNA. J Biol Chem. 1979 Sep 25;254(18):9220–9226. [PubMed] [Google Scholar]
- Dunn J. J., Studier F. W. Complete nucleotide sequence of bacteriophage T7 DNA and the locations of T7 genetic elements. J Mol Biol. 1983 Jun 5;166(4):477–535. doi: 10.1016/s0022-2836(83)80282-4. [DOI] [PubMed] [Google Scholar]
- Firestone G. L., Payvar F., Yamamoto K. R. Glucocorticoid regulation of protein processing and compartmentalization. Nature. 1982 Nov 18;300(5889):221–225. doi: 10.1038/300221a0. [DOI] [PubMed] [Google Scholar]
- Friedman D. I., Olson E. R. Evidence that a nucleotide sequence, "boxA," is involved in the action of the NusA protein. Cell. 1983 Aug;34(1):143–149. doi: 10.1016/0092-8674(83)90144-7. [DOI] [PubMed] [Google Scholar]
- Goff C. G., Setzer J. ADP ribosylation of Escherichia coli RNA polymerase is nonessential for bacteriophage T4 development. J Virol. 1980 Jan;33(1):547–549. doi: 10.1128/jvi.33.1.547-549.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldfarb A. Changes in the promoter range of RNA polymerase resulting from bacteriophage T4-induced modification of core enzyme. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3454–3458. doi: 10.1073/pnas.78.6.3454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldfarb A., Palm P. Control of promoter utilization by bacteriophage T4-induced modification of RNA polymerase alpha subunit. Nucleic Acids Res. 1981 Oct 10;9(19):4863–4878. doi: 10.1093/nar/9.19.4863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golomb M., Chamberlin M. Characterization of T7-specific ribonucleic acid polymerase. IV. Resolution of the major in vitro transcripts by gel electrophoresis. J Biol Chem. 1974 May 10;249(9):2858–2863. [PubMed] [Google Scholar]
- Greenblatt J., Li J. Interaction of the sigma factor and the nusA gene protein of E. coli with RNA polymerase in the initiation-termination cycle of transcription. Cell. 1981 May;24(2):421–428. doi: 10.1016/0092-8674(81)90332-9. [DOI] [PubMed] [Google Scholar]
- Herman R. E., Haas N., Snustad D. P. Identification of the bacteriophage T4 unf ( = alc) gene product, a protein involved in the shutoff of host transcription. Genetics. 1984 Oct;108(2):305–317. doi: 10.1093/genetics/108.2.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvitz H. R. Bacteriophage T4 mutants deficient in alteration and modification of the Escherichia coli RNA polymerase. J Mol Biol. 1974 Dec 25;90(4):739–750. doi: 10.1016/0022-2836(74)90537-3. [DOI] [PubMed] [Google Scholar]
- Horvitz H. R. Control by bacteriophage T4 of two sequential phosphorylations of the alpha subunit of Escherichia coli RNA polymerase. J Mol Biol. 1974 Dec 25;90(4):727–738. doi: 10.1016/0022-2836(74)90536-1. [DOI] [PubMed] [Google Scholar]
- Jin D. J., Cashel M., Friedman D. I., Nakamura Y., Walter W. A., Gross C. A. Effects of rifampicin resistant rpoB mutations on antitermination and interaction with nusA in Escherichia coli. J Mol Biol. 1988 Nov 20;204(2):247–261. doi: 10.1016/0022-2836(88)90573-6. [DOI] [PubMed] [Google Scholar]
- Jin D. J., Walter W. A., Gross C. A. Characterization of the termination phenotypes of rifampicin-resistant mutants. J Mol Biol. 1988 Jul 20;202(2):245–253. doi: 10.1016/0022-2836(88)90455-x. [DOI] [PubMed] [Google Scholar]
- Kassavetis G. A., Chamberlin M. J. Pausing and termination of transcription within the early region of bacteriophage T7 DNA in vitro. J Biol Chem. 1981 Mar 25;256(6):2777–2786. [PubMed] [Google Scholar]
- Kingston R. E., Nierman W. C., Chamberlin M. J. A direct effect of guanosine tetraphosphate on pausing of Escherichia coli RNA polymerase during RNA chain elongation. J Biol Chem. 1981 Mar 25;256(6):2787–2797. [PubMed] [Google Scholar]
- Koerner J. F., Snustad D. P. Shutoff of host macromolecular synthesis after T-even bacteriophage infection. Microbiol Rev. 1979 Jun;43(2):199–223. doi: 10.1128/mr.43.2.199-223.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutter E. M., Bradley D., Schenck R., Guttman B. S., Laiken R. Bacteriophage T4 alc gene product: general inhibitor of transcription from cytosine-containing DNA. J Virol. 1981 Dec;40(3):822–829. doi: 10.1128/jvi.40.3.822-829.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutter E., Beug A., Sluss R., Jensen L., Bradley D. The production of undegraded cytosine-containing DNA by bacteriophage T4 in the absence of dCTPase and endonucleases II and IV, and its effects on T4-directed protein synthesis. J Mol Biol. 1975 Dec 25;99(4):591–607. doi: 10.1016/s0022-2836(75)80174-4. [DOI] [PubMed] [Google Scholar]
- Kutter E., Drivdahl R., Rand K. Identification and characterization of the alc gene product of bacteriophage T4. Genetics. 1984 Oct;108(2):291–304. doi: 10.1093/genetics/108.2.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEHMAN I. R., PRATT E. A. On the structure of the glucosylated hydroxymethylcytosine nucleotides of coliphages T2, T4, and T6. J Biol Chem. 1960 Nov;235:3254–3259. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Landick R., Yanofsky C. Stability of an RNA secondary structure affects in vitro transcription pausing in the trp operon leader region. J Biol Chem. 1984 Sep 25;259(18):11550–11555. [PubMed] [Google Scholar]
- Leirmo S., Harrison C., Cayley D. S., Burgess R. R., Record M. T., Jr Replacement of potassium chloride by potassium glutamate dramatically enhances protein-DNA interactions in vitro. Biochemistry. 1987 Apr 21;26(8):2095–2101. doi: 10.1021/bi00382a006. [DOI] [PubMed] [Google Scholar]
- Levin J. R., Chamberlin M. J. Mapping and characterization of transcriptional pause sites in the early genetic region of bacteriophage T7. J Mol Biol. 1987 Jul 5;196(1):61–84. doi: 10.1016/0022-2836(87)90511-0. [DOI] [PubMed] [Google Scholar]
- Lisitsyn N. A., Sverdlov E. D., Moiseyeva E. P., Danilevskaya O. N., Nikiforov V. G. Mutation to rifampicin resistance at the beginning of the RNA polymerase beta subunit gene in Escherichia coli. Mol Gen Genet. 1984;196(1):173–174. doi: 10.1007/BF00334112. [DOI] [PubMed] [Google Scholar]
- Lue N. F., Kornberg R. D. Accurate initiation at RNA polymerase II promoters in extracts from Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1987 Dec;84(24):8839–8843. doi: 10.1073/pnas.84.24.8839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mailhammer R., Yang H. L., Reiness G., Zubay G. Effects of bacteriophage T4-induced modification of Escherichia coli RNA polymerase on gene expression in vitro. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4928–4932. doi: 10.1073/pnas.72.12.4928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik S., Dimitrov M., Goldfarb A. Initiation of transcription by bacteriophage T4-modified RNA polymerase independently of host sigma factor. J Mol Biol. 1985 Sep 5;185(1):83–91. doi: 10.1016/0022-2836(85)90184-6. [DOI] [PubMed] [Google Scholar]
- Morton D., Kutter E. M., Guttman B. S. Synthesis of T4 DNA and bacteriophage in the absence of dCMP hydroxymethylase. J Virol. 1978 Oct;28(1):262–269. doi: 10.1128/jvi.28.1.262-269.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nierman W. C., Chamberlin M. J. Studies of RNA chain initiation by Escherichia coli RNA polymerase bound to T7 DNA. Direct analysis of the kinetics and extent of RNA chain initiation at T7 promoter A1. J Biol Chem. 1979 Aug 25;254(16):7921–7926. [PubMed] [Google Scholar]
- Pabo C. O., Sauer R. T. Protein-DNA recognition. Annu Rev Biochem. 1984;53:293–321. doi: 10.1146/annurev.bi.53.070184.001453. [DOI] [PubMed] [Google Scholar]
- Pearson R. E., Snyder L. Shutoff of lambda gene expression by bacteriophage T4: role of the T4 alc gene. J Virol. 1980 Jul;35(1):194–202. doi: 10.1128/jvi.35.1.194-202.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratner D. The interaction bacterial and phage proteins with immobilized Escherichia coli RNA polymerase. J Mol Biol. 1974 Sep 15;88(2):373–383. doi: 10.1016/0022-2836(74)90488-4. [DOI] [PubMed] [Google Scholar]
- Rohrer H., Zillig W., Mailhammer R. ADP-ribosylation of DNA-dependent RNA polymerase of Escherichia coli by an NAD+: protein ADP-ribosyltransferase from bacteriophage T4. Eur J Biochem. 1975 Dec 1;60(1):227–238. doi: 10.1111/j.1432-1033.1975.tb20995.x. [DOI] [PubMed] [Google Scholar]
- Schmidt M. C., Chamberlin M. J. Amplification and isolation of Escherichia coli nusA protein and studies of its effects on in vitro RNA chain elongation. Biochemistry. 1984 Jan 17;23(2):197–203. doi: 10.1021/bi00297a004. [DOI] [PubMed] [Google Scholar]
- Snustad D. P., Haas N., Oppenheimer D. G. The bacteriophage T4 regulatory protein gpunf/alc binds to DNA in the absence of RNA polymerase. J Virol. 1986 Dec;60(3):1145–1147. doi: 10.1128/jvi.60.3.1145-1147.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snustad D. P., Tigges M. A., Parson K. A., Bursch C. J., Caron F. M., Koerner J. F., Tutas D. J. Identification and preliminary characterization of a mutant defective in the bacteriophage T4-induced unfolding of the Escherichia coli nucleoid. J Virol. 1976 Feb;17(2):622–641. doi: 10.1128/jvi.17.2.622-641.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder L., Gold L., Kutter E. A gene of bacteriophage T4 whose product prevents true late transcription on cytosine-containing T4 DNA. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3098–3102. doi: 10.1073/pnas.73.9.3098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder L., Jorissen L. Escherichia coli mutations that prevent the action of the T4 unf/alc protein map in an RNA polymerase gene. Genetics. 1988 Feb;118(2):173–180. doi: 10.1093/genetics/118.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder L., Jorissen L. Molecular proof that bacteriophage T4 alc and unf genes are the same gene. J Bacteriol. 1986 Nov;168(2):833–838. doi: 10.1128/jb.168.2.833-838.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens A. Deoxyribonucleic acid dependent ribonucleic acid polymerases from two T4 phage-infected systems. Biochemistry. 1974 Jan 29;13(3):493–503. doi: 10.1021/bi00700a015. [DOI] [PubMed] [Google Scholar]
- Stevens A. Inhibition of DNA-enzyme binding by an RNA polymerase inhibitor from T4 phage-infected Escherichia coli. Biochim Biophys Acta. 1977 Mar 2;475(1):193–196. doi: 10.1016/0005-2787(77)90355-0. [DOI] [PubMed] [Google Scholar]
- Stevens A. New small polypeptides associated with DNA-dependent RNA polymerase of Escherichia coli after infection with bacteriophage T4. Proc Natl Acad Sci U S A. 1972 Mar;69(3):603–607. doi: 10.1073/pnas.69.3.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WYATT G. R., COHEN S. S. The bases of the nucleic acids of some bacterial and animal viruses: the occurrence of 5-hydroxymethylcytosine. Biochem J. 1953 Dec;55(5):774–782. doi: 10.1042/bj0550774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiggs J. L., Bush J. W., Chamberlin M. J. Utilization of promoter and terminator sites on bacteriophage T7 DNA by RNA polymerases from a variety of bacterial orders. Cell. 1979 Jan;16(1):97–109. doi: 10.1016/0092-8674(79)90191-0. [DOI] [PubMed] [Google Scholar]
- Williams K. P., Kassavetis G. A., Esch F. S., Geiduschek E. P. Identification of the gene encoding an RNA polymerase-binding protein of bacteriophage T4. J Virol. 1987 Feb;61(2):597–599. doi: 10.1128/jvi.61.2.597-599.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams K. P., Müller R., Rüger W., Geiduschek E. P. Overproduced bacteriophage T4 gene 33 protein binds RNA polymerase. J Bacteriol. 1989 Jun;171(6):3579–3582. doi: 10.1128/jb.171.6.3579-3582.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson G. G., Tanyashin V. I., Murray N. E. Molecular cloning of fragments of bacteriophage T4 DNA. Mol Gen Genet. 1977 Nov 14;156(2):203–214. doi: 10.1007/BF00283493. [DOI] [PubMed] [Google Scholar]
- Wood W. B. Host specificity of DNA produced by Escherichia coli: bacterial mutations affecting the restriction and modification of DNA. J Mol Biol. 1966 Mar;16(1):118–133. doi: 10.1016/s0022-2836(66)80267-x. [DOI] [PubMed] [Google Scholar]