Abstract
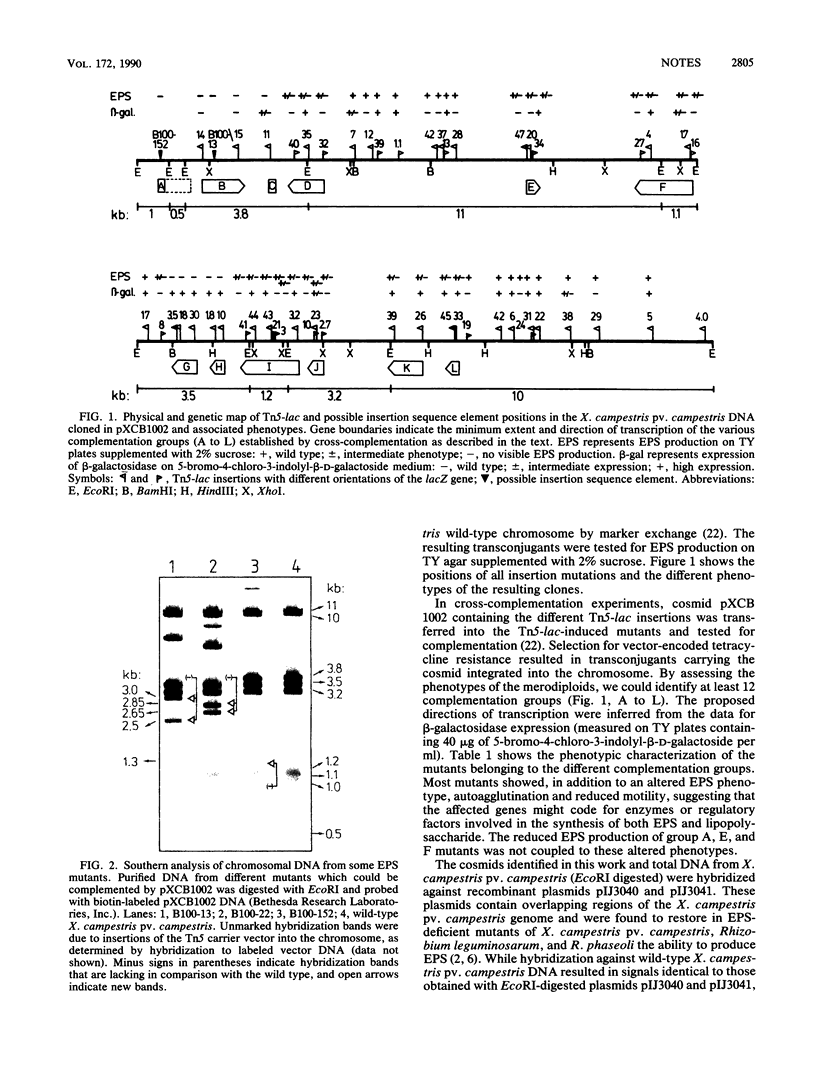
Cosmid clones able to restore exopolysaccharide production in possibly insertion sequence element-induced surface mutants of Xanthomonas campestris pv. campestris were isolated. By fragment-specific Tn5-lac mutagenesis of one of the cosmids, pXCB1002, a new DNA region which is involved in exopolysaccharide biosynthesis and which is organized into at least 12 complementation groups was identified.
Full text
PDF
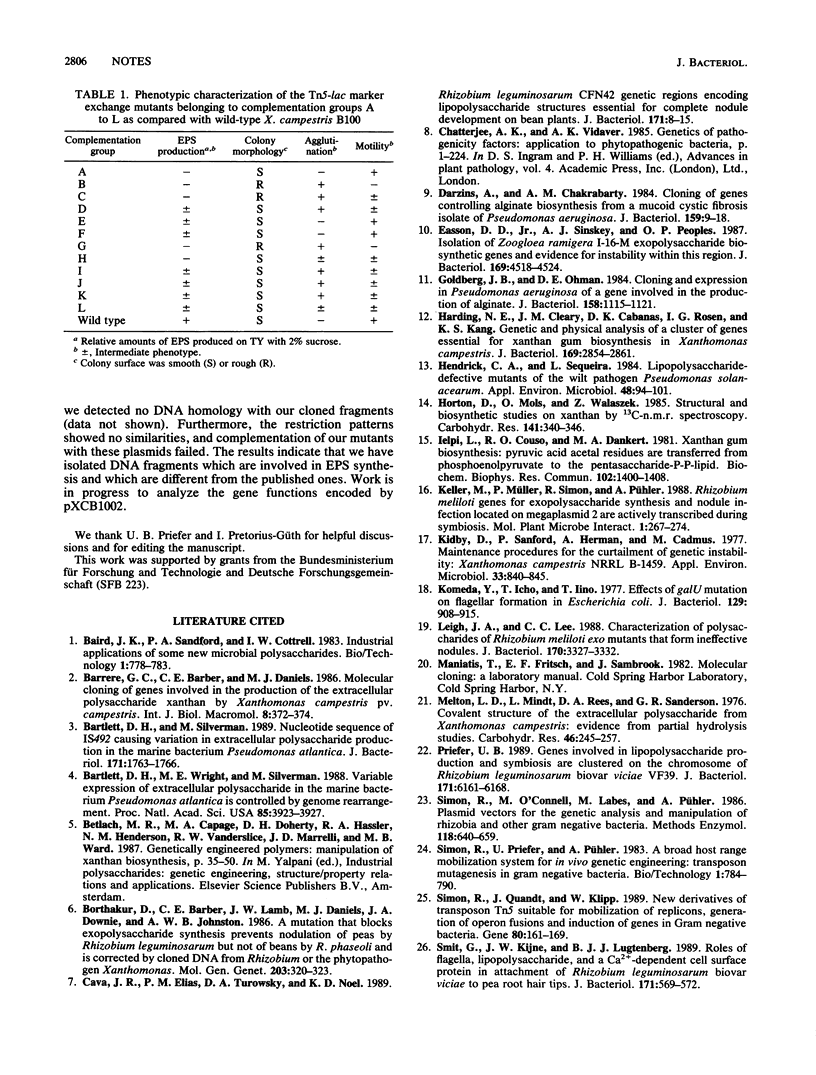

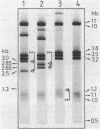
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartlett D. H., Silverman M. Nucleotide sequence of IS492, a novel insertion sequence causing variation in extracellular polysaccharide production in the marine bacterium Pseudomonas atlantica. J Bacteriol. 1989 Mar;171(3):1763–1766. doi: 10.1128/jb.171.3.1763-1766.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett D. H., Wright M. E., Silverman M. Variable expression of extracellular polysaccharide in the marine bacterium Pseudomonas atlantica is controlled by genome rearrangement. Proc Natl Acad Sci U S A. 1988 Jun;85(11):3923–3927. doi: 10.1073/pnas.85.11.3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cava J. R., Elias P. M., Turowski D. A., Noel K. D. Rhizobium leguminosarum CFN42 genetic regions encoding lipopolysaccharide structures essential for complete nodule development on bean plants. J Bacteriol. 1989 Jan;171(1):8–15. doi: 10.1128/jb.171.1.8-15.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darzins A., Chakrabarty A. M. Cloning of genes controlling alginate biosynthesis from a mucoid cystic fibrosis isolate of Pseudomonas aeruginosa. J Bacteriol. 1984 Jul;159(1):9–18. doi: 10.1128/jb.159.1.9-18.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easson D. D., Jr, Sinskey A. J., Peoples O. P. Isolation of Zoogloea ramigera I-16-M exopolysaccharide biosynthetic genes and evidence for instability within this region. J Bacteriol. 1987 Oct;169(10):4518–4524. doi: 10.1128/jb.169.10.4518-4524.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg J. B., Ohman D. E. Cloning and expression in Pseudomonas aeruginosa of a gene involved in the production of alginate. J Bacteriol. 1984 Jun;158(3):1115–1121. doi: 10.1128/jb.158.3.1115-1121.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrick C. A., Sequeira L. Lipopolysaccharide-Defective Mutants of the Wilt Pathogen Pseudomonas solanacearum. Appl Environ Microbiol. 1984 Jul;48(1):94–101. doi: 10.1128/aem.48.1.94-101.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ielpi L., Couso R. O., Dankert M. A. Pyruvic acid acetal residues are transferred from phosphoenolpyruvate to the pentasaccharide-P-P-lipid. Biochem Biophys Res Commun. 1981 Oct 30;102(4):1400–1408. doi: 10.1016/s0006-291x(81)80167-2. [DOI] [PubMed] [Google Scholar]
- Kidby D., Sandford P., Herman A., Cadmus M. Maintenance procedures for the curtailment of genetic instability: Xanthomonas campestris NRRL B-1459. Appl Environ Microbiol. 1977 Apr;33(4):840–845. doi: 10.1128/aem.33.4.840-845.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komeda Y., Icho T., Iino T. Effects of galU mutation on flagellar formation in Escherichia coli. J Bacteriol. 1977 Feb;129(2):908–915. doi: 10.1128/jb.129.2.908-915.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leigh J. A., Lee C. C. Characterization of polysaccharides of Rhizobium meliloti exo mutants that form ineffective nodules. J Bacteriol. 1988 Aug;170(8):3327–3332. doi: 10.1128/jb.170.8.3327-3332.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melton L. D., Mindt L., Rees D. A., Sanderson G. R. Covalent structure of the extracellular polysaccharide from Xanthomonas campestris: evidence from partial hydrolysis studies. Carbohydr Res. 1976 Feb;46(2):245–257. doi: 10.1016/s0008-6215(00)84296-2. [DOI] [PubMed] [Google Scholar]
- Priefer U. B. Genes involved in lipopolysaccharide production and symbiosis are clustered on the chromosome of Rhizobium leguminosarum biovar viciae VF39. J Bacteriol. 1989 Nov;171(11):6161–6168. doi: 10.1128/jb.171.11.6161-6168.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon R., O'Connell M., Labes M., Pühler A. Plasmid vectors for the genetic analysis and manipulation of rhizobia and other gram-negative bacteria. Methods Enzymol. 1986;118:640–659. doi: 10.1016/0076-6879(86)18106-7. [DOI] [PubMed] [Google Scholar]
- Simon R., Quandt J., Klipp W. New derivatives of transposon Tn5 suitable for mobilization of replicons, generation of operon fusions and induction of genes in gram-negative bacteria. Gene. 1989 Aug 1;80(1):161–169. doi: 10.1016/0378-1119(89)90262-x. [DOI] [PubMed] [Google Scholar]
- Smit G., Kijne J. W., Lugtenberg B. J. Roles of flagella, lipopolysaccharide, and a Ca2+-dependent cell surface protein in attachment of Rhizobium leguminosarum biovar viciae to pea root hair tips. J Bacteriol. 1989 Jan;171(1):569–572. doi: 10.1128/jb.171.1.569-572.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland I. W. Biosynthesis and composition of gram-negative bacterial extracellular and wall polysaccharides. Annu Rev Microbiol. 1985;39:243–270. doi: 10.1146/annurev.mi.39.100185.001331. [DOI] [PubMed] [Google Scholar]
- Thorne L., Tansey L., Pollock T. J. Clustering of mutations blocking synthesis of xanthan gum by Xanthomonas campestris. J Bacteriol. 1987 Aug;169(8):3593–3600. doi: 10.1128/jb.169.8.3593-3600.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whatley M. H., Hunter N., Cantrell M. A., Hendrick C., Keegstra K., Sequeira L. Lipopolysaccharide Composition of the Wilt Pathogen, Pseudomonas solanacearum: CORRELATION WITH THE HYPERSENSITIVE RESPONSE IN TOBACCO. Plant Physiol. 1980 Mar;65(3):557–559. doi: 10.1104/pp.65.3.557. [DOI] [PMC free article] [PubMed] [Google Scholar]