Abstract
Collagens act as important signaling molecules regulating vascular smooth muscle cell responses during arterial wound repair. Discoidin domain receptors (DDRs) are a novel class of receptor tyrosine kinases that bind to several collagens and stimulate matrix metalloproteinase (MMP) production, but little is known about their expression and function in the vasculature. We posited a critical role for the DDRs controlling smooth muscle cell migration and proliferation and thus repair following arterial injury. Smooth muscle cells were isolated from the aortas of mice with a targeted deletion of the DDR1 gene (DDR1-null) and studied in culture using models that mimic critical steps in neointimal thickening. Our studies suggest that DDR1 plays an important role in regulating attachment to collagen, chemotaxis, proliferation, and MMP production in smooth muscle cells. Following mechanical injury to the carotid arteries, cross-sectional area of the neointima was significantly lower in DDR1-null mice than in wild-type mice. There was also a significant decrease in collagen deposition in the injured arteries of the DDR1-null mice. Our results support the hypothesis that DDR1 plays an important role as a collagen receptor, mediating intimal thickening after vascular injury.
Introduction
Atherosclerosis and restenosis are characterized by the development of a thickened neointimal layer in the blood vessel wall. Smooth muscle cells (SMCs) are activated after arterial injury and contribute to neointimal lesion development through proliferation, migration, and ECM synthesis. Recent research suggests that the ECM is not simply an inert scaffold, but instead there are dynamic interactions between cells and matrix that contribute to SMC responses. After arterial injury, SMCs synthesize the fibrillar type I and III collagens (1, 2) and the short-chain type VIII collagen (3–6). Recently, the discoidin domain receptor tyrosine kinases (DDRs) were shown to function as collagen receptors and to increase matrix metalloproteinase (MMP) production in a fibrosarcoma cell line (7, 8). The MMPs (including MMP-1, MMP-2, MMP-3, MMP-9, and MT1-MMP) are upregulated after injury and facilitate SMC migration in the vessel wall (9–12). Given the role of the DDRs in mediating interactions with collagen and stimulating MMP synthesis, we posit here that the DDRs are important mediators of the SMC response to injury.
The DDRs are distinguished by an extracellular domain of 160 amino acids that is homologous to the Dictyostelium discoideum protein discoidin-I (13). There are two distinct gene products, DDR1 and DDR2, and DDR1 appears in three alternative splice variants, 1a, 1b, and 1c (13). DDR1 is widely expressed during embryonic development and in adult tissues, particularly in the epithelium of skin, kidney, gut, and brain, and the splice variant DDR1b increases considerably during postnatal development. DDR2 is restricted to skeletal muscle, heart, and connective tissues (13). Strikingly high levels of DDR1 and 2 are seen in fast-growing invasive mammary, ovarian, and lung tumors (14), in keeping with the increased proliferative rates and MMP production in these tumors. To date, one abstract has reported DDR1 and DDR2 expression in atherosclerotic lesions of nonhuman primates fed a high-cholesterol diet (15); however, nothing is known about the function of DDRs in the vascular system. In the current study we have examined DDR1, the most widely expressed DDR in adult mammals.
The arterial collagens that are upregulated after injury can be classified broadly into two categories based on their structure; fibril-forming (type I and III) and short-chain (type VIII) collagens. Tissue-culture studies show that type I collagen affects SMC growth and migration (16–18). Type VIII collagen is a short-chain collagen expressed during active remodeling in angiogenesis (19), embryonic development of the heart (20), and glomerulonephritis (21). We and others have shown that it is dramatically upregulated following experimental arterial injury (3–5) and in human atherosclerotic plaques (6), and we have shown recently that it acts as a chemotactic factor and stimulates MMP synthesis by SMCs in vitro (22).
To investigate the role of the DDR1 in atherosclerosis and restenosis, we examined expression of the DDR1 receptor in the injured rat carotid artery, performed in vitro studies with SMCs isolated from DDR1 knockout mice, and measured the response to arterial injury in DDR1-null mice.
Methods
All chemicals were obtained from Sigma Chemical Co. (St. Louis, Missouri, USA) unless otherwise stated.
Transient expression of DDR1 in HEK 293 cells and Western blot analysis for DDR1 phosphorylation.
Rat type I collagen was purchased from Collaborative Biomedical Products (Bedford, Massachusetts, USA). Type VIII collagen was purified from bovine Descemet’s membrane using our methods described previously (22). Human embryonic kidney fibroblast 293 cells were obtained from American Type Culture Collection (Manassas, Virginia, USA). A cDNA for human DDR1 was overexpressed using a vector that has been described earlier (14). Semiconfluent 293 cells were transfected by calcium-phosphate precipitation with a cytomegalovirus-based expression vector. Sixteen hours later, cells were transferred to serum-free media for an additional 24 hours. Cells were stimulated with 10 μg/ml type I or type VIII collagen for 90 minutes, lysed with 1% Triton X-100, 50 mM HEPES (pH 7.5), 150 mM NaCl, 1.5 mM MgCl2, 5 mM EGTA, 5 mM EDTA, 10% glycerol, 10 mM NaF, 1 mM PMSF, 1 mM Na-orthovanadate, and 10 μg/ml aprotinin. The cellular lysates were then centrifuged 10 minutes at 4°C and 16,100 g, and aliquots of the supernatant were subjected to SDS-PAGE and Western blotting with monoclonal anti-phosphotyrosine Ab (4G10; Upstate Biotechnology Inc., Lake Placid, New York, USA) diluted 1:500 in 50 mM Tris (pH 7.5), 150 mM NaCl, 5 mM EDTA, 0.05% gelatin. Western blots were developed using horseradish peroxidase–coupled secondary Ab (Bio-Rad Laboratories, Hercules, California, USA) and enhanced chemiluminescence (Amersham Life Science, Oakville, Ontario, Canada). The blots were stripped, then reprobed with a polyclonal Ab raised against the extracellular domain of DDR1 (amino acids 29–186), using bacterial expressed antigens (7).
DDR1 expression after balloon-catheter injury of the rat carotid artery.
Animal experiments were approved and carried out in accordance with the guidelines of the Canadian Council on Animal Care. A total of 80 male Sprague-Dawley rats (3–4 months old) (Charles River, Constant, Quebec, Canada) were used in all experiments. Rats were anesthetized by intraperitoneal injection of xylazine (4.6 mg/kg body weight, Rompum; Bayer Inc., Toronto, Ontario, Canada) and ketamine (70 mg/kg body weight, Ketaset; Ayerst Veterinarian Laboratories, Guelph, Ontario, Canada), and subject to balloon-catheter injury of the left carotid artery as described previously (9). For Northern blot analysis of DDR1 mRNA expression, rats were sacrificed at 1, 2, 4, 8, 14, and 28 days after injury. The carotids were harvested, stripped of adventitia, then pooled for RNA extraction (6–8 vessels per time point), and total cellular RNA (15 μg/lane) was used to prepare Northern blots according to our methods published previously (9). Photographs of methylene blue–stained Northern blots were used to demonstrate equal loading of the lanes based on density of the 28s and 18s ribosomal RNA bands. The blots were hybridized with a cDNA probe for mouse DDR1 (14) labeled with 32P-dCTP by random primer extension (Multi-Prime; Amersham). The hybridized blots were used to expose Hyperfilm-MP (Amersham).
A second series of rats was subjected to carotid injury, and the vessels were harvested at 1 hour, 6 hours, 1, 4, 7, and 14 days after injury for Western blots (the adventitia was stripped from the vessels after harvest). Four rats per time point were analyzed. Arterial protein extracts were prepared by pulverizing the arteries under liquid nitrogen, then lysed in buffer containing 1% SDS, 1 mM PMSF, and 10 mg/ml leupeptin in 50 mM Tris (pH 7.6). These extracts were used to generate Western blots loaded with 10 μg total cellular protein in each lane, which were then probed with a rabbit polyclonal Ab against the human DDR1 carboxy-terminus (amino acids 894–913) at a concentration of 0.4 μg/ml (Santa Cruz Biotechnology Inc., Santa Cruz, California, USA). The Western blots were detected using enhanced chemiluminescence as described above. The blot shown (see Figure 1) is a representative sample of four separate Western blots.
Figure 1.
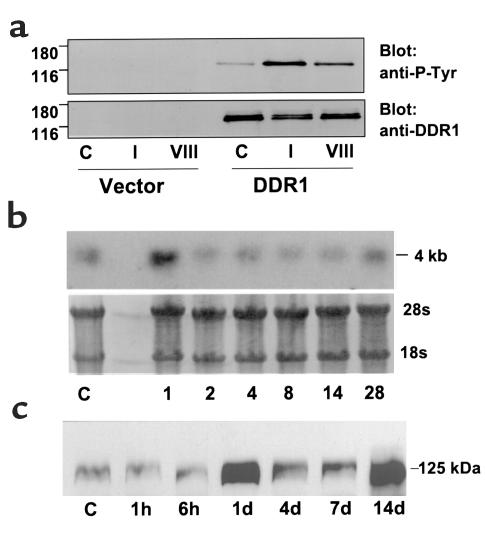
(a) DDR1 was activated by type I and type VIII collagen. HEK 293 cells were transfected with vector alone or DDR1 cDNA. The cells were incubated with plain media (C), 10 μg/ml type I collagen (I), or 10 μg/ml type VIII collagen (VIII) for 90 minutes, then cell lysates were collected and subjected to Western blotting. The same blots were sequentially probed with anti-phosphotyrosine (anti-P-Tyr) Ab (upper) and with DDR1 Ab (lower). (b) DDR1 mRNA expression was increased after injury of the rat carotid artery. Northern blot with RNA extracted from control carotids (C) and from carotids at various days after injury, then probed with a cDNA against mouse DDR1. Lower photograph is the methylene-blue–stained Northern blot showing 28s and 18s ribosomal RNA bands demonstrating equal loading in the lanes. (c) A Western blot, with arterial protein extracts from control carotids (C) and carotids taken at various times after injury, was probed with anti-human DDR1 Ab.
A third series of rats was subjected to balloon-catheter injury. The rats were sacrificed at 2-, 4- ,and 14-day time points, and the carotids were perfusion fixed at constant physiologic pressure with 4% paraformaldehyde (four rats per time point). The vessels were excised and embedded in paraffin blocks, and two cross-sections were cut at positions 1 and 2 cm upstream of the carotid bifurcation. The slides were incubated with the rabbit polyclonal anti-human DDR1 at a concentration of 0.4 μg/ml for 1 hour at 37°C. The secondary Ab, biotinylated donkey anti-rabbit IgG (Jackson ImmunoResearch Laboratories Inc., West Grove, Pennsylvania, USA) at a dilution of 1:1000, was applied for 1 hour at room temperature. Sections stained with secondary Ab alone in the absence of primary Ab served as negative controls for the immunostaining. Microscopic images of the cross-sections (×600) were captured using a Nikon Coolpix 950 digital camera (Nikon Canada Inc., Mississauga, Ontario, Canada).
Generation of DDR1-null mice.
A targeting vector was constructed using the pPNT plasmid with a 750-bp EcoR1/BamH1 fragment and a 2.3-kb Xho1/Not1 fragment of the murine genomic DDR1 cloned to either side of the Neo cassette. In the mouse DDR1 locus, the EcoR1 site is located in the 5′-untranslated region and the BamH1 site 12 bp upstream of the start codon. The Xho1 site is located in the 12th intron and the Not1 site in the last coding exon. The linearized vector was electroporated into R1 embryonic stem (ES) cells, and transfected cells were selected with G418 and ganciclovir. Targeted ES cell lines were aggregated with blastocysts from ICR mice (Charles River) and implanted into pseudopregnant ICR females. The resultant chimeras were mated with 129/sv females, and heterozygous offspring were intercrossed into 129/sv, as well as ICR and C57Bl6 backgrounds, to generate inbred and outbred DDR1-null mice. We did not observe any strain-dependent differences in the phenotype of DDR1-null mice. Experiments were performed with homozygous DDR1-null mice, and homozygous wild-type littermates were used as controls.
Cell dispersion and primary culture of adult mouse aortic SMCs.
SMCs were isolated from the aortas of 36 DDR1-null mice and 36 wild-type (mixture of 129/sv and C57Bl6 strains) littermates. The aortas were excised, then flushed with sterile DMEM containing 1% HEPES and 1% penicillin-streptomycin (Life Technologies Inc., Gaithersburg, Maryland, USA) to remove blood. Using a dissecting microscope, the vessels were cut open longitudinally, and the endothelial cells and adventitia were scraped off with a scalpel blade. The vessels (12 vessels were pooled per dish) were then transferred to fresh dispersion media: DMEM with 1% HEPES, 1% penicillin-streptomycin, 1.8 mg/ml collagenase type I (Worthington Biochemical Co., Freehold, New Jersey, USA), 0.3 mg/ml elastase type III, 0.44 mg/ml soybean trypsin-inhibitor type I, and 2 mg/ml BSA. The tissue was minced with a scalpel blade, maintained at 37°C, and mechanically dispersed by pipetting vigorously every 10 minutes for approximately 1 hour until the cells were dispersed. After dispersion, the cells were suspended in 50 ml DMEM with 10% FCS and 2% penicillin-streptomycin to neutralize the enzymes. The cells were centrifuged at 652 g for 5 minutes, then resuspended in 3 ml DMEM with 10% FCS and 2% penicillin-streptomycin and cultured in flasks. Cells were used for experiments between passages three and ten. Western blots containing cell lysates were probed with the anti-DDR1 Ab to ensure that the SMCs from the DDR1-null mice were negative and with a polyclonal anti-mouse β1-integrin Ab (Chemicon International, Temecula, California, USA) to assess the level of β1-integrin expression. Levels of DDR2 in the SMCs were measured using RT-PCR.
SMC attachment assay.
SMC attachment assays for wild-type and DDR1-null SMCs were performed by coating 96-well tissue-culture plates overnight with 50 μl of 100-nM solutions of type I or type VIII collagen or 10 nM solutions of fibronectin or vitronectin. These concentrations of substrate were chosen based on previous studies showing that they promoted 50% maximum attachment of rat SMCs (22, 23). SMCs were suspended in serum-free DMEM containing 1 mg/ml BSA, plated at a density of 60,000 cells per well, and allowed to attach at 37°C for 4 hours. Nonadherent cells were rinsed off with PBS, the remaining attached cells fixed with 4% paraformaldehyde, then stained with 0.5% toluidine blue, and the absorbance of the solution in the well was measured in a microtiter plate reader (Molecular Devices, Sunnyvale, California, USA) at 595 nm. The attachment experiments were performed in triplicate and repeated three times.
SMC growth assay: thymidine incorporation.
Twenty-four–well tissue-culture plates were coated overnight with 100 μl of a 100-nM solution of type I or type VIII collagen, followed by seeding of wild-type or DDR1-null SMCs at a density of 60,000 cells/well. After incubation with DMEM containing 10% FCS for 8 hours to allow cell attachment, the cells were incubated in serum-free DMEM for 16 hours to synchronize the cells in Go phase. Then FCS (10%) and a tracer amount of [3H]thymidine (2 μCi/well, specific activity 3.15 Bq/mmol; Amersham), was added and the cells were incubated for another 16-hour period. Then the cells were washed three times with PBS and fixed with ice-cold 10% (wt/vol) trichloroacetic acid. [3H]thymidine radioactivity in the precipitate was measured in an LS-6500 liquid-scintillation counter (Beckman Instruments Inc., Fullerton, California, USA). DDR1-null and wild-type SMCs attached equally well to the plate after 8 hours in serum, presumably because vitronectin and fibronectin from the serum provide a substrate for efficient attachment.
SMC growth curves.
Wild-type or DDR1-null SMCs were seeded at a density of 60,000 cells/well in six-well tissue-culture plates coated with 2 ml of a solution of 100 nM type I collagen. Cells were allowed to attach in 10% serum, then serum starved to arrest growth as described above. The media was then changed to DMEM with 10% FCS serum, and the cells were incubated for periods of up to 4 days. At 1, 2, 3, and 4 days, cells were removed from the wells using 0.05% trypsin and counted using a hemocytometer. SMC number at 3 days after plating on fibronectin (100 nM) or in response to 10 ng/ml of PDGF-BB were assessed as controls. We chose to stimulate cell growth with FCS because it contains multiple growth factors and cytokines and because it is a relevant growth stimulus for SMCs in the endothelium-denuded vessel wall. However, the effects of FCS may be confounded by the presence of attachment factors such as fibronectin and vitronectin, so PDGF-BB was used as an additional control.
SMC-migration assay.
Migration assays were performed using chemotaxis chambers (Becton Dickinson and Co., Franklin Lakes, New Jersey, USA) with 84,000 SMCs plated per well and 200 nM type I or type VIII collagen used as a chemoattractant suspended in 250 μl of serum-free DMEM in the bottom chamber. These concentrations of collagen were chosen based on our previous studies showing 50% maximal stimulation of migration for rat SMCs. The cells were allowed to migrate for 4 hours, then the cells were fixed with 4% paraformaldehyde, and the cells that had migrated to the bottom of the filter were stained with Diff-Quick (Dade Behring Inc., Newark, Delaware, USA). Migration was quantitated by counting the number of cells in five random ×200 fields/filter and expressed as the average number of cells per field. For a positive control we measured migration toward 10 ng/ml PDGF-BB suspended in serum-free DMEM in the bottom chamber. Attachment of wild-type and DDR1-null SMCs to the top of the filters was equivalent. Each experiment was performed in duplicate and repeated three times.
Gelatin zymograms for MMP activity.
Wild-type or DDR1-null SMCs were seeded at a density of 60,000 cells/well in 96-well plates coated with 50 μl of 0, 100, or 1000 nM solutions of type I collagen. The cells were incubated with 10% FCS for 8 hours to allow cell attachment, then the cells were serum starved for 16 hours, followed by a change in media and incubation with 10% FCS for 24 hours. Twenty microliters of conditioned media from each well was loaded on an SDS-PAGE gel containing 0.1% gelatin as a substrate for MMP activity. Zymograms were processed and stained as we have described previously (9).
Wire injury of the mouse carotid artery.
Eight DDR1-null mice (four males, four females) and six wild-type (mixture of 129/sv and C57Bl6 strains) (three males, three females) littermate controls were used in these experiments. Surgery was performed with the surgeon blinded to the genotype of the mice to avoid bias. Mice were anesthetized by intraperitoneal injection of xylazine (10 mg/kg body weight) (Rompum; Bayer Inc.) and ketamine (150 mg/kg BW) (Ketaset; Ayerst Veterinary Laboratories). The mouse carotids were injured using a protocol published previously (24) with the following modifications. A copper wire 0.3 mm in diameter was inserted into the left external carotid artery, then passed through into the common carotid. The common carotid was injured by pulling the wire through the carotid four times, rotating the wire 90° with each pass. The mice were sacrificed 14 days after injury, a catheter placed in the left ventricle to retrograde perfuse the carotids, first with lactated Ringer’s injection solution (Baxter Inc., Toronto, Ontario, Canada), followed by perfusion with 0.1 M phosphate-buffered 4% paraformaldehyde at constant physiologic pressure. The common carotid arteries were embedded in paraffin blocks, then cross-sections from the middle of the vessel were stained with hematoxylin and eosin, and images were captured using a Nikon E600 microscope and a Coolpix 950 digital camera (Nikon Canada Inc.). Intimal, medial, and adventitial cross-sectional areas, the number of cell nuclei in the intima, and external elastic lamina (EEL) perimeter were measured using Simple Software (C-Imaging Systems, Mars, Pennsylvania, USA). Intimal cell density was calculated as the number of nuclei per square micrometer of intimal area. Additional sections were stained with picrosirius red dye to label collagen (25) and examined using polarized-light microscopy to detect birefringent collagen fibrils in the vessel wall. Simple Software was used to semiquantitatively analyze collagen deposition by measuring the percentage of intimal area that was positively stained with picrosirius red.
Statistical analysis.
Differences in cell attachment, proliferation, and migration between wild-type and DDR1-null SMCs were analyzed using Student’s t test. Differences in intimal, medial, and adventitial cross-sectional area, EEL perimeter, and intimal cell density between wild-type and DDR1-null mouse carotids after injury were analyzed using Student’s t test.
Results
Type I and type VIII collagen activate DDR phosphorylation.
Upon binding to collagen, DDRs are autophosphorylated by tyrosine kinase activity present in the receptor cytoplasmic tail. Therefore, measurement of receptor tyrosine phosphorylation is commonly used to demonstrate binding and activation of the DDRs. To determine whether two collagen types produced in abundance in the injured vessel wall could activate DDR1, we transiently transfected HEK 293 cells to overexpress DDR1 and stimulated the cells with collagen. A band with molecular weight of 125 kDa was tyrosine phosphorylated after stimulation with 10 μg/ml type I collagen and was also phosphorylated to a lesser degree after stimulation with 10 μg/ml type VIII collagen (Figure 1a). There was no detectable expression of DDR1 in HEK 293 cells transfected with vector alone. There was some autophosphorylation of DDR1 in the absence of ligand, as indicated by the phosphotyrosine band visible in the control lane (labeled C).
DDR1 mRNA and protein were increased after balloon-catheter injury of the rat carotid artery.
We next measured expression of DDR1 mRNA and protein in the balloon-injured rat carotid artery. Northern blots containing total RNA extracted from control and balloon-injured rat carotids were probed with a mouse DDR1 cDNA, and we detected a band of approximately 4 kb. DDR1 expression was transiently increased 1 day after balloon-catheter injury of the rat carotid artery; expression returned to control levels between 2 and 14 days and was increased again at 28 days after injury (Figure 1b). DDR1 protein levels were assessed by Western blots prepared from arterial extracts taken at various times after injury and bound with a polyclonal Ab against human DDR1, which cross-reacts with a band at 125 kDa in the rat extracts. This is comparable with the molecular weights reported for human and mouse DDR1. The level of DDR1 protein was dramatically increased in the injured rat carotid 1 day after balloon injury and was elevated again at 14 days after injury (Figure 1c).
Immunostaining of rat carotid cross-sections with anti-human DDR1 Ab showed cell-associated staining and a similar time course for receptor expression. There was minimal staining for DDR1 in the media and strong staining in the adventitia of uninjured control carotids (Figure 2a). This suggests that adventitial fibroblasts, and to a much lesser extent medial SMCs, express DDR1, while the endothelium is negative. DDR1 staining was increased in medial SMCs immediately subjacent to the lumen 2 days after injury (Figure 2b). There was intense staining of the migrating neointimal SMCs 4 days after injury (Figure 2c), which persisted throughout the thickness of the intima at 14 days (Figure 2d). Sections incubated in the absence of the anti- DDR1 Ab did not show staining (Figure 2e).
Figure 2.
DDR1 immunostaining was increased after injury of the rat carotid artery. Vessel cross-sections from uninjured control carotid arteries (a) and carotid arteries at 2 days (b), 4 days (c), and 14 days (d) after balloon-catheter injury were stained with anti-DDR1 Ab. (e) A cross-section was stained in the absence of the anti-DDR1 Ab. ×600.
DDR1-null SMCs showed reduced attachment to collagen, but normal attachment to fibronectin and vitronectin.
To examine the role of DDR1 in SMC interactions with collagen, cultured SMCs from wild-type or DDR1-null mice were used in attachment, proliferation, and migration assays. The attachment of DDR1-null SMCs to 100 nM type I collagen was reduced by 55% compared with wild-type SMCs (P < 0.0001), and attachment to 100 nM type VIII collagen was reduced by 72% (P < 0.0001) (Figure 3). Attachment to 10 nM fibronectin (P = 0.06) or vitronectin (P = 0.35) was not significantly affected, demonstrating that the deficit in attachment was specific for the collagen ligands. We used RT-PCR to show that expression of DDR2 was equivalent in DDR1-null and wild-type SMCs and used Western blots to show that the level of β1-integrin expression was also equivalent (not shown).
Figure 3.
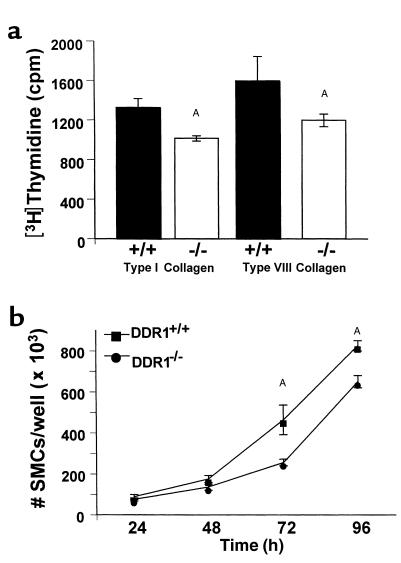
SMC attachment to collagen was reduced in the absence of DDR1. SMCs from DDR1+/+ mice (filled bars) or DDR1–/– mice (open bars) were allowed to attach for 4 hours to plates coated with 10 nM fibronectin or vitronectin or 100 nM type I collagen or type VIII collagen. Attachment was assessed by staining the cells with toluidine blue and measuring optical density at 595 nm. Experiments were done in triplicate and repeated three times. AValue from DDR1–/– SMCs was significantly less than the value from wild-type SMCs.
DDR1-null SMCs showed reduced proliferation on collagen.
Incorporation of [3H]thymidine was used as an index of SMC proliferation in cells plated on collagen and stimulated to grow with 10% FCS. Thymidine incorporation by DDR1-null SMCs plated on type I collagen (1014 ± 27 cpm) was significantly less than wild-type SMCs (1326 ± 89 cpm; P = 0.0005) (Figure 4a). Thymidine incorporation on type VIII collagen was also significantly reduced in DDR1-null SMCs (P = 0.043) (Figure 4a). Furthermore, growth of DDR1-null SMCs plated on collagen was attenuated compared with wild-type controls, as evidenced by a significant decrease in the number of SMCs that accumulated after 3 and 4 days in culture with 10% FCS added to the media (Figure 4b). By contrast, there was no significant difference between the growth of wild-type and DDR1-null SMCs plated on 100 nM fibronectin. There were 383 × 103 ± 8 × 103 wild-type cells/well and 378 × 103 ± 8 × 103 DDR1-null cells/well after 3 days of serum stimulation. There was no difference in the growth of wild-type and DDR1-null SMCs stimulated with PDGF-BB (the number of cells per well was 483 × 103 ± 6 × 103 and 500 × 103 ± 7 × 103, respectively).
Figure 4.
Cell proliferation on collagen was less in DDR1–/– SMCs compared with DDR1+/+ SMCs. (a) SMCs from DDR1+/+ mice (filled bars) or DDR1–/– mice (open bars) were stimulated to grow with 10% FCS and incubated with [3H]thymidine on wells coated with 100 nM type I collagen (left) or type VIII collagen (right). AValue from DDR1–/– SMCs was significantly less than value from wild-type SMCs. (b) Number of SMCs per well after plating on 100 nM type I collagen and growing in DMEM containing 10% FCS for 1–4 days. Squares, DDR1+/+ SMCs; circles, DDR1–/– SMCs. Experiments were done in triplicate and repeated three times. AValue from DDR1–/– SMCs was significantly less than value from wild-type SMCs.
We attempted to assess the rate of apoptosis after serum starvation by staining cells with propidium iodide and counting cells with evidence of nuclear fragmentation, but we observed no nuclear fragmentation in either SMC population.
DDR1-null SMCs showed reduced migration toward collagen.
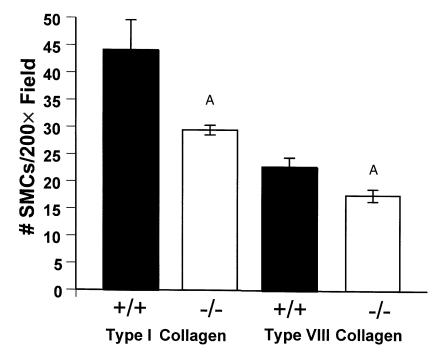
Modified Boyden chambers were used to assess the chemotactic response of SMCs to collagen or PDGF-BB. The migration of DDR1-null SMCs toward type I or type VIII collagen was significantly reduced by 33% (P = 0.01) and 23% (P = 0.005), respectively, compared with wild-type SMCs (Figure 5). By contrast, the migration of DDR1-null SMCs in response to 10 ng/ml PDGF-BB was equivalent to wild-type controls (data not shown).
Figure 5.
SMC migration was reduced in the absence of DDR1. SMCs from DDR1+/+ mice (filled bars) or DDR1–/– mice (open bars) were stimulated to migrate for 4 hours in chemotaxis chambers with 200 nM type I collagen (left) or type VIII collagen (right) added to DMEM in the bottom of the chamber. Migration was quantified by staining and counting the number of cells that migrated to the bottom of the filter. Experiments were done in triplicate and repeated three times. AValues from DDR1–/– SMCs were significantly less than values from wild-type SMCs.
DDR1-null SMCs had reduced MMP expression.
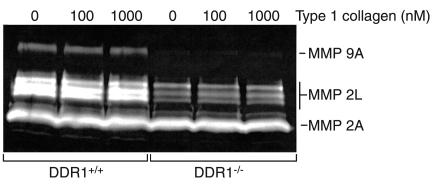
Gelatin zymograms run with conditioned media from wild-type SMCs revealed the presence of active MMP-9 (Mr 83 kDa) as well as three forms of latent MMP-2 (Mr 72, 70, and 68 kDa) and two forms of active MMP-2 (Mr 61 and 58 kDa) (Figure 6). By contrast, DDR1-null SMCs expressed less proteinase activity. There was no MMP-9 and much less latent MMP-2, while the amount of active MMP-2 was equivalent to wild-type cells (Figure 6). We observed no dose response to increasing collagen substrate concentration in either cell type. The size and identification of lytic bands in the zymogram gels was similar to that reported for extracts of injured mouse carotid arteries (26).
Figure 6.
DDR1-null SMCs produced no MMP-9 activity and less MMP-2 activity compared with wild-type cells. Gelatin zymogram containing conditioned media from wild-type (DDR1+/+) or DDR1-null SMCs (DDR1–/–) plated on wells precoated with 0, 100, or 1000 nM type I collagen. MMP-9A, MMP-9 active; MMP-2L, MMP-2 zymogen; MMP-2A, MMP-2 active.
Targeted disruption of the DDR1 gene reduced neointimal growth after carotid injury.
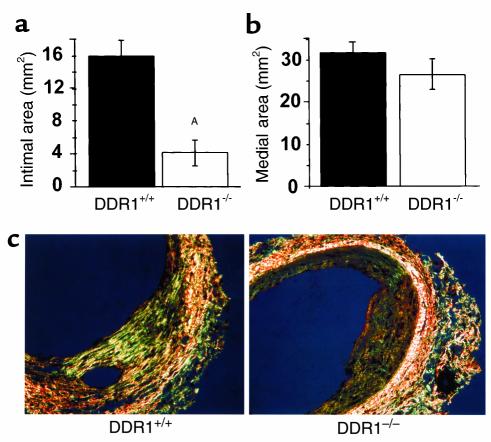
Intimal thickening after vascular injury was dramatically attenuated in DDR1-null mice compared with wild-type controls. Intimal cross-sectional area was reduced from 15.88 ± 1.88 μm2 in wild-type mice to 4.10 ± 1.58 μm2 in DDR1-null mice at 14 days after injury (P = 0.0006) (Figure 7a). There were no significant differences in medial area (Figure 7b), adventitial area, or EEL perimeter (not shown) between wild-type and knockout mice.
Figure 7.
Intimal area was significantly decreased after injury of the DDR1-null mouse carotid compared with wild-type. (a) Cross-sectional area of intima measured 14 days after carotid injury. (b) Cross-sectional area of media measured 14 days after carotid injury. AIntimal area in DDR1–/– mice was significantly less than intimal area in wild-type mice. (n = 6 and 8 for DDR1+/+ and DDR1–/– groups, respectively.) (c) There was less collagen deposition in the carotid arterial intima of the DDR1-null mouse. Picrosirius red staining and polarized light microscopy were used to assess the deposition of birefringent collagen in the vessel wall. ×400.
Interestingly, the density of cells in the intima was almost two times higher in DDR1-null mice, being 15 cells/μm2 intimal cross-section area compared with 8 cells/μm2 in wild-type mice. This suggested a decrease in matrix accumulation in the DDR1-null mouse compared with wild-type. Using picrosirius red stain and polarized-light microscopy to visualize collagen birefringence, we noted a substantial decrease in the deposition of collagen fibrils in the intima of the DDR1-null mice compared with wild-type controls (Figure 7c). When measured by digital image analysis, the percentage of area of the intima containing collagen was 23.9 ± 6.0% in wild-type mice compared with 6.4 ± 3.2% in DDR1-null mice.
Discussion
SMC proliferation, migration, and collagen synthesis contribute to the development of neointimal thickening in atherosclerosis and restenosis. We have used an experimental model of balloon-catheter injury of the rat carotid artery that mimics critical features of this process to study expression of the collagen-receptor DDR1. We chose to use this model because the kinetics of the response to injury are very well-defined; SMC proliferation peaks within 1 or 2 days after injury (27), and the first SMCs begin migrating to the neointima 3–4 days after injury (28). MMP activity is increased over the same time frame (9) and is necessary for SMC migration from media to intima (10, 29). This allowed us to show that DDR1 was expressed in concurrence with the proliferation and migration of medial SMCs; mRNA expression and protein production for DDR1 was increased by 1 day after injury. Migrating SMCs immediately subjacent to the lumen of the vessel at 2 days after injury stained positive, and at 4 and 14 days neointimal SMCs remained positive for DDR1.
Collagen expression has been studied in the rat model, and we show in the current study that expression of DDR1 parallels the increased mRNA expression and immunostaining for types I, III, and VIII collagen (1, 3, 4). Furthermore, our in vitro studies show that all three collagens are ligands for the DDRs (7). There is a growing body of experimental evidence suggesting that these collagen types control SMC responses. Type I collagen functions directly as a chemotactic factor for SMCs (18), and migration can be inhibited by blocking collagen synthesis and assembly in vitro (30). In addition, type I collagen potentiates PDGF-BB–stimulated SMC migration (16, 31). With respect to growth, plating human vascular SMCs on monomeric type I collagen stimulates cell proliferation, while fibrillar collagen maintains cell quiescence (17), suggesting that the structure of collagen may play an important role in determining function. Culture of SMCs in three-dimensional collagen gels stimulates MMP production, a step necessary for cell invasion through the three-dimensional matrices (32). Furthermore, we recently demonstrated that type VIII collagen stimulates SMC migration and production of MMP-2 and MMP-9 in vitro (22). Several of these studies implicate the collagen-binding integrins, α2β1 and α1β1, in mediating these effects. By contrast, very little is known about the function of the DDRs, therefore we have examined their role in mediating SMC interactions with collagen.
To address this, we used DDR1-null mice generated by targeted homologous gene deletion (33). The DDR1-null mice have a reduced body weight before puberty in both sexes, which persists after puberty in the females but not the males. In addition, there are defects in implantation and mammary gland development. Blood vessels in the DDR1-null mice appear normal in size and structure. Aortic SMCs were isolated for study in vitro using models that mimic critical steps in neointimal development. It is necessary for a cell to attach to matrix to gain traction for migration (34), and we found that SMC attachment to both type I and type VIII collagen was dramatically reduced in the absence of DDR1. By contrast, the DDR1-null SMCs attached normally to fibronectin and vitronectin, ruling out a nonspecific deficit in cell attachment and confirming the specificity of DDR1 as a collagen receptor. Migration toward collagen in a chemotaxis chamber was significantly attenuated in the absence of DDR1; however, the response to the chemotactic growth factor PDGF-BB was not affected. Serum-stimulated SMC thymidine uptake and growth were also significantly reduced in DDR1-null SMCs plated on collagen. By contrast, serum-stimulated growth of cells plated on fibronectin and growth in response to soluble PDGF-BB was equivalent in wild-type and DDR1-null SMCs. The differences in growth rates, though statistically significant, were relatively small. This might be due to the presence of fibronectin in the serum, which would stimulate the proliferation of both cell types.
The recent discovery that DDR2 activation stimulated MMP-1 production in a fibrosarcoma cell line (7) and the knowledge that MMPs are critically important for SMC migration and invasion led us to investigate the hypothesis that DDR1 could stimulate an increase in MMP production in vascular SMCs. We found that DDR1-null SMCs had no MMP-9 activity and far less MMP-2 activity than wild-type cells. This appears to be independent of substrate since the differences persisted even in cells plated on plastic. We focused on MMP-2 and MMP-9 because our previous work and that of others showed that these molecules were dramatically upregulated early after vascular injury and play important roles in SMC migration (9, 10, 12, 26).
To the best of our knowledge, these studies are the first to demonstrate a functional role for the DDR1 in cell attachment, proliferation, and migration. Several collagen-binding receptors are known to be expressed on SMCs, including α1β1 and α2β1 integrins, as well as the DDR2. It appears that they cannot compensate, since the responses of DDR1-null SMCs were impaired despite the presence of normal levels of the other collagen receptors. The functions of DDR1 may also be important during development and in other pathologies. In support of this, DDRs are overexpressed in several rapidly growing and invasive tumors (13). In a previous publication we showed that dominant-negative DDR1 inhibits myoblast differentiation in vitro (35). Finally, impaired axonal migration and outgrowth have been observed in cerebellar granule neurons overexpressing a truncated, dominant-negative variant of DDR1 (36). Taken together, the data are consistent with an important role for the DDRs in controlling cell growth and migration.
Very little is known about the signaling pathways activated by the DDRs. Ligand binding induces receptor dimerization and transphosphorylation of tyrosine in the cytoplasmic domain (35). The DDR1b isoform binds the adapter protein Shc, however extracellular signal-related kinase (ERK) is not activated (7). The cytoplasmic domain of DDR1 includes consensus binding sequences for the SH2 domains of Nck, GAP, and the p85 subunit of PI3-kinase; however, these pathways have not been investigated (13). Furthermore, there may be cross-talk between the DDRs and the collagen-binding integrins, α2β1 and α1β1. A recent paper shows that DDR1 can be activated by soluble collagen in the absence of functional β1-integrin receptors (35). This does not rule out the possibility of cross-talk in downstream signaling pathways.
The in vitro results with DDR1-null SMCs led us to predict a critical impairment in neointimal formation after vascular injury. Indeed, we found that neointimal development was significantly reduced in DDR1-null mice compared with wild-type mice. In addition we found a dramatic decrease in collagen deposition in the neointima of DDR1-null mice. DDR1 could therefore function as a collagen sensor that is activated by various types of collagen and is an essential part of the cellular machinery that triggers ECM degradation and renewal. However, there may be other ligands for the DDRs in addition to the collagens.
Experiments are underway to define the time course and magnitude of the response to injury in the DDR1-null mouse carotid and to extend this work to other models of atherosclerosis. Mechanical injury of the mouse carotid artery is a simple model providing valuable information on SMC responses after arterial injury; however, there is little inflammation with this injury. We stained carotid sections with mAb’s against macrophages (Mac-3) and neutrophils (Ly-6G); however, only three to four inflammatory cells per cross-section were detected in only two out of six wild-type mouse carotids, and no inflammatory cells were detected in the DDR1-null mouse carotids (unpublished data). By contrast, apoE-null mice exhibit a progressive accumulation of macrophages, SMCs, and lipid-laden foam cells in the subendothelial intimal space, with development of a fibrous cap and necrotic core at the center of the plaque, closely resembling the human atherosclerotic lesion (37). In the future, we will cross the DDR1-null mice with apoE-null mice to investigate the function of the DDRs in a murine model of atherosclerosis.
In conclusion, we found that DDR1 expression was upregulated early after balloon injury of the carotid artery in coincidence with cell proliferation, migration, and type I and VIII collagen expression. In vitro, SMCs from DDR1-null mice had reduced attachment to, reduced proliferation on, and reduced migration toward collagen, and reduced MMP activity. These in vitro endpoints provide mechanistic insight into the basis of the impairment in neointimal thickening following arterial injury in DDR1-null mice. Furthermore, collagen matrix accumulation was reduced in the intima after injury of the carotid artery. Although we are still far from having a full picture of the DDR-signaling pathways, this study is the first, to our knowledge, to suggest that the DDR1 plays an important role in mediating lesion growth after vascular injury by binding to collagen deposited in the ECM.
Acknowledgments
This work was funded by Heart and Stroke Foundation of Ontario grant B4009, and Medical Research Council of Canada grant MT-37847 to M. Bendeck. M. Bendeck was a Research Scholar of the Heart and Stroke Foundation of Canada. W. Vogel was a recipient of a fellowship of the Deutsche Forschungsgemeinschaft. The authors are extremely grateful to Tony Pawson for providing the DDR1-null mice used in this work. We would also like to acknowledge Mansoor Husain for assistance with the development of the mouse arterial-injury model, and David Courtman for help with polarized-light microscopy. Lee Adamson provided the facilities to perform the mouse surgeries. The technical assistance of Diane Mulholland was greatly appreciated.
References
- 1.Majesky MW, Lindner V, Twardzik DR, Schwartz SM, Reidy MA. Production of transforming growth factor β1 during repair of arterial injury. J Clin Invest. 1991;88:904–910. doi: 10.1172/JCI115393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Strauss BH, et al. Extracellular matrix remodeling after balloon angioplasty injury in a rabbit model of restenosis. Circ Res. 1994;75:650–658. doi: 10.1161/01.res.75.4.650. [DOI] [PubMed] [Google Scholar]
- 3.Bendeck MP, et al. Differential expression of α1 type VIII collagen in injured, platelet-derived growth factor-BB stimulated rat carotid arteries. Circ Res. 1996;79:524–531. doi: 10.1161/01.res.79.3.524. [DOI] [PubMed] [Google Scholar]
- 4.Sibinga NES, et al. Collagen VIII is expressed by vascular smooth muscle cells in response to vascular injury. Circ Res. 1997;80:532–541. doi: 10.1161/01.res.80.4.532. [DOI] [PubMed] [Google Scholar]
- 5.Plenz G, Dorszewski A, Breithardt G, Robenek H. Expression of type VIII collagen after cholesterol diet and injury in the rabbit model of atherosclerosis. Arterioscler Thromb Vasc Biol. 1999;19:1201–1209. doi: 10.1161/01.atv.19.5.1201. [DOI] [PubMed] [Google Scholar]
- 6.MacBeath JRE, Kielty CM, Shuttleworth CA. Type VIII collagen is a product of vascular smooth-muscle cells in development and disease. Biochem J. 1996;319:993–998. doi: 10.1042/bj3190993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vogel W, Gish GD, Alves F, Pawson T. The discoidin domain receptor tyrosine kinases are activated by collagen. Mol Cell. 1997;1:13–23. doi: 10.1016/s1097-2765(00)80003-9. [DOI] [PubMed] [Google Scholar]
- 8.Shrivastava A, et al. An orphan receptor tyrosine kinase family whose members serve as nonintegrin collagen receptors. Mol Cell. 1997;1:25–34. doi: 10.1016/s1097-2765(00)80004-0. [DOI] [PubMed] [Google Scholar]
- 9.Bendeck MP, Zempo N, Clowes AW, Galardy RE, Reidy MA. Smooth muscle cell migration and matrix metalloproteinase expression after arterial injury in the rat. Circ Res. 1994;75:539–545. doi: 10.1161/01.res.75.3.539. [DOI] [PubMed] [Google Scholar]
- 10.Bendeck MP, Irvin C, Reidy MA. Inhibition of matrix metalloproteinase activity inhibits smooth muscle cell migration but not neointimal thickening after arterial injury. Circ Res. 1996;78:38–43. doi: 10.1161/01.res.78.1.38. [DOI] [PubMed] [Google Scholar]
- 11.Southgate KM, et al. Upregulation of basement membrane-degrading metalloproteinase secretion after balloon injury of pig carotid arteries. Circ Res. 1996;79:1177–1187. doi: 10.1161/01.res.79.6.1177. [DOI] [PubMed] [Google Scholar]
- 12.Godin D, Ivan E, Johnson C, Magid R, Galis ZS. Remodeling of carotid artery is associated with increased expression of matrix metalloproteinases in mouse blood flow cessation model. Circulation. 2000;102:2861–2866. doi: 10.1161/01.cir.102.23.2861. [DOI] [PubMed] [Google Scholar]
- 13.Vogel W. Discoidin domain receptors: structural relations and functional implications. FASEB J. 1999;13(Suppl.):S77–S82. doi: 10.1096/fasebj.13.9001.s77. [DOI] [PubMed] [Google Scholar]
- 14.Alves F, et al. Distinct structural characteristics of discoidin I subfamily receptor tyrosine kinases and complementary expression in human cancer. Oncogene. 1995;10:609–618. [PubMed] [Google Scholar]
- 15.Carragher, N.O., Vogel, W., and Raines, E. 2000. Integrin-associated MMP activity and remodeling of fibrillar type I collagen by vascular smooth muscle cells. Potential regulation by discoidin domain receptor activation. Keystone Symposium on Joint Regulation of Signaling Pathways by Integrins and Growth Factors. p. 92. (Abstr.)
- 16.Skinner MP, Raines EW, Ross R. Dynamic expression of α1β1 and α2β1 integrin receptors by human vascular smooth muscle cells: α2β1 integrin is required for chemotaxis across type I collagen-coated membranes. Am J Pathol. 1994;145:1070–1081. [PMC free article] [PubMed] [Google Scholar]
- 17.Koyama H, Raines EW, Bornfeldt KE, Roberts JM, Ross R. Fibrillar collagen inhibits arterial smooth muscle cell proliferation through regulation of Cdk2 inhibitors. Cell. 1996;87:1069–1078. doi: 10.1016/s0092-8674(00)81801-2. [DOI] [PubMed] [Google Scholar]
- 18.Gotwals PJ, et al. The α1β1 integrin is expressed during neointima formation in rat arteries and mediates collagen matrix reorganization. J Clin Invest. 1996;97:2469–2477. doi: 10.1172/JCI118693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iruela-Arispe ML, Diglio CA, Sage EH. Modulation of extracellular matrix proteins by endothelial cells undergoing angiogenesis in vitro. Arterioscler Thromb. 1991;11:805–815. doi: 10.1161/01.atv.11.4.805. [DOI] [PubMed] [Google Scholar]
- 20.Sage H, Iruela-Arispe ML. Type VIII collagen in murine development. Association with capillary formation in vitro. Ann NY Acad Sci. 1990;580:17–31. doi: 10.1111/j.1749-6632.1990.tb17914.x. [DOI] [PubMed] [Google Scholar]
- 21.Rosenblum ND. The mesangial matrix in the normal and sclerotic glomerulus. Kidney Int. 1994;45:S73–S77. [PubMed] [Google Scholar]
- 22.Hou G, Mulholland D, Gronska MA, Bendeck MP. Type VIII collagen stimulates smooth muscle cell migration and matrix metalloproteinase synthesis after arterial injury. Am J Pathol. 2000;156:467–476. doi: 10.1016/S0002-9440(10)64751-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liaw L, Almeida M, Hart CE, Schwartz SM, Giachelli CM. Osteopontin promotes vascular cell adhesion and spreading and is chemotactic for smooth muscle cells in vitro. Circ Res. 1994;74:214–224. doi: 10.1161/01.res.74.2.214. [DOI] [PubMed] [Google Scholar]
- 24.Lindner V, Fingerle J, Reidy MA. Mouse model of arterial injury. Circ Res. 1993;73:792–796. doi: 10.1161/01.res.73.5.792. [DOI] [PubMed] [Google Scholar]
- 25.Sweat F, Puchtler H, Rosenthal SI. Sirius red F3BA as a stain for connective tissues. Arch Pathol. 1964;78:69–72. [PubMed] [Google Scholar]
- 26.Lijnen HR, et al. Function of the plasminogen/plasmin and matrix metalloproteinase systems after vascular injury in mice with targeted inactivation of fibrinolytic system genes. Arterioscler Thromb Vasc Biol. 1998;18:1035–1045. doi: 10.1161/01.atv.18.7.1035. [DOI] [PubMed] [Google Scholar]
- 27.Clowes AW, Reidy MA, Clowes MM. Kinetics of cellular proliferation after arterial injury. I. Smooth muscle growth in the absence of endothelium. Lab Invest. 1983;49:327–333. [PubMed] [Google Scholar]
- 28.Jackson CL, Raines E, Ross R, Reidy MA. Role of endogenous platelet-derived growth factor in arterial smooth muscle cell migration after balloon catheter injury. Arterioscler Thromb. 1993;13:1218–1226. doi: 10.1161/01.atv.13.8.1218. [DOI] [PubMed] [Google Scholar]
- 29.Zempo N, Koyama N, Kenagy RD, Lea HJ, Clowes A. Regulation of vascular smooth muscle cell migration and proliferation in vitro and in injured rat arteries by a synthetic matrix metalloproteinase inhibitor. Arterioscler Thromb Vasc Biol. 1996;16:28–33. doi: 10.1161/01.atv.16.1.28. [DOI] [PubMed] [Google Scholar]
- 30.Rocnik EF, Chan BMC, Pickering JG. Evidence for a role of collagen synthesis in arterial smooth muscle cell migration. J Clin Invest. 1998;101:1889–1898. doi: 10.1172/JCI1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stringa E, Knauper V, Murphy G, Gavrilovic J. Collagen degradation and platelet-derived growth factor stimulate the migration of vascular smooth muscle cells. J Cell Sci. 2000;113:2055–2064. doi: 10.1242/jcs.113.11.2055. [DOI] [PubMed] [Google Scholar]
- 32.Langholz O, et al. Collagen and collagenase gene expression in three-dimensional collagen lattices are differentially regulated by α1β1 and α2β1 integrins. J Cell Biol. 1995;131:1903–1915. doi: 10.1083/jcb.131.6.1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vogel, W., Aszodi, A., Alves, F., and Pawson, T. 2001. Discoidin domain receptor 1 tyrosine kinase has an essential role in mammary gland development. Mol. Cell. Biol. In press. [DOI] [PMC free article] [PubMed]
- 34.Palecek SP, Loftus JC, Ginsberg MH, Lauffenburger DA, Horwitz AF. Integrin-ligand binding properties govern cell migration speed through cell-substratum adhesiveness. Nature. 1997;385:537–540. doi: 10.1038/385537a0. [DOI] [PubMed] [Google Scholar]
- 35.Vogel W, et al. Discoidin domain receptor 1 is activated independently of β1 integrin. J Biol Chem. 2000;275:5779–5784. doi: 10.1074/jbc.275.8.5779. [DOI] [PubMed] [Google Scholar]
- 36.Bhatt RS, Tomada T, Fang Y, Hatten ME. Discoidin domain receptor 1 functions in axon extension of cerebellar granule neurons. Genes Dev. 2000;14:2216–2228. doi: 10.1101/gad.821600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hofker MH, Van Vlijmen BJM, Havekes LM. Transgenic mouse models to study the role of APOE in hyperlipidemia and atherosclerosis. Atherosclerosis. 1998;137:1–11. doi: 10.1016/s0021-9150(97)00266-9. [DOI] [PubMed] [Google Scholar]