Abstract
O2 deprivation can produce many devastating clinical conditions such as myocardial infarct and stroke. The molecular mechanisms underlying the inherent tissue susceptibility or tolerance to O2 lack are, however, not well defined. Since the fruit fly, Drosophila melanogaster, is extraordinarily tolerant to O2 deprivation, we have performed a genetic screen in the Drosophila to search for loss-of-function mutants that are sensitive to low O2. Here we report on the genetic and molecular characterization of one of the genes identified from this screen, named hypnos-2. This gene encodes a Drosophila pre-mRNA adenosine deaminase (dADAR) and is expressed almost exclusively in the adult central nervous system. Disruption of the dADAR gene results in totally unedited sodium (Para), calcium (Dmca1A), and chloride (DrosGluCl-α) channels, a very prolonged recovery from anoxic stupor, a vulnerability to heat shock and increased O2 demands, and neuronal degeneration in aged flies. These data clearly demonstrate that, through the editing of ion channels as targets, dADAR, for which there are mammalian homologues, is essential for adaptation to altered environmental stresses such as O2 deprivation and for the prevention of premature neuronal degeneration.
Introduction
Human tissues vary markedly in their ability to tolerate O2 deprivation. In the central nervous system, for example, neurons are extremely vulnerable to limited O2 supply while astrocytes are more tolerant to the same stress (1, 2). Compared with renal tubular cells, skeletal muscle cells are very tolerant of O2 deprivation. Of particular interest also are recent studies showing that human tumor cells can be very tolerant of anoxia (0% of oxygen) (3, 4), raising the distinct possibility that genetic manipulation can induce anoxia tolerance in human cells. However, it has been very difficult to probe the genetic pathways that render cells more resistant to O2 deprivation in vertebrate animals in vivo.
Drosophila melanogaster, a well-studied genetic model organism, has been used to dissect the molecular and genetic basis for a number of complex biological processes (5–7). Our laboratory has shown previously that this organism is very tolerant to O2 deprivation, and this tolerance is genetically endowed (8–10). A direct search for fruit fly mutants that are sensitive to lack of O2 has led us to identify several loss-of-function mutants that are much more sensitive to anoxia. Of these mutants, two have been located in the same locus (10) and named hypnos-2P and hypnos-2L. Compared with hypnos-2L, hypnos-2P has a much stronger sensitivity to total lack of O2. We therefore focused our work on this allele.
Methods
Fly stocks.
Wild-type flies were Canton-S (C-S). The fly stock used for transgenic experiments was w1118. A fly line carrying 32B-GAL4 on the third chromosome was used for rescue experiments. Deficient flies of df(1)S39 and df(1)A94 were obtained from Bloomington Fly Stocks Center (Bloomington, Indiana, USA); deficient st472 was kindly provided by Igor Zhimulev (Institute of Cytology and Genetics, Novosibirsk, Russia). Balancer chromosomes used were FM7c, Adv, CyO, TM3 sb, and TM6B Hu.
Recovery from anoxia.
The procedure for inducing anoxia and measuring recovery time has been described previously (8, 10). Briefly, groups of 10–15 adult flies, age 3–4 days, were placed in a specially designed chamber and exposed to anoxia (O2 concentration = 0% with administration of 100% N2) for 5 minutes before allowing recovery in room air (O2 = 20.8%). After the introduction of N2 in the chamber, flies became uncoordinated within approximately 30 seconds, fell to the bottom of the container, stopped moving, and stayed motionless for the rest of the anoxia period. Recovery time was measured as the latency between the end of a 5-minute anoxia treatment and the time point when the fly recovered.
O2 consumption.
In a closed system consisting of a specially designed Plexiglas jar (130 cm3 volume), O2 consumption of Drosophila is measured in room air and in a low-O2 condition (1.5% O2) using an O2 probe (DO-O51; Cameron Instrument Co., Port Aransas, Texas, USA). Acclimatization time before any baseline measurement was 30 minutes. Baseline recordings were then made for 1 hour and were followed by O2-consumption measurements in hypoxic periods lasting for 60 minutes. In each trial, at least 1,000 flies were used. O2 consumption was calculated from the drop in O2 concentration in the jar.
Heat-shock response.
In this experiment, the vials containing adult flies (age 3–4 days, 10–15 flies per vial) were placed in a 37°C water bath for certain periods. In six trials, 214 of hypnos-2P and 229 of C-S flies were used. Numbers of dead flies were recorded at the time points of 2.0, 2.5, 3.0, 3.5, 4.0, 4.5, 5.0, 5.5, and 6.0 hours during incubation.
Life span.
Vials containing ten males and ten females from hypnos-2P or control, newly enclosed C-S flies were placed at 25°C. In each group, 200 flies were used. The flies were passaged onto fresh food every 2–3 days. Dead flies were counted with each vial change.
Genetic and cytological mapping of hypnos-2P.
Our previous work has shown that hypnos-2P is located between 1B5 and 5B (10). To refine the cytological location of this mutation, complementation tests with several deficiencies were carried out. Briefly, male hypnos-2P flies were crossed to virgin females with df(1)A94/FM7c, df(1)S39/FM7c, and df(1)st472/FM7c. From these crosses, female progenies carrying hypnos-2P and a deficiency (hypnos-2P/deficiency) were subjected to an anoxia test.
Southern blot analysis.
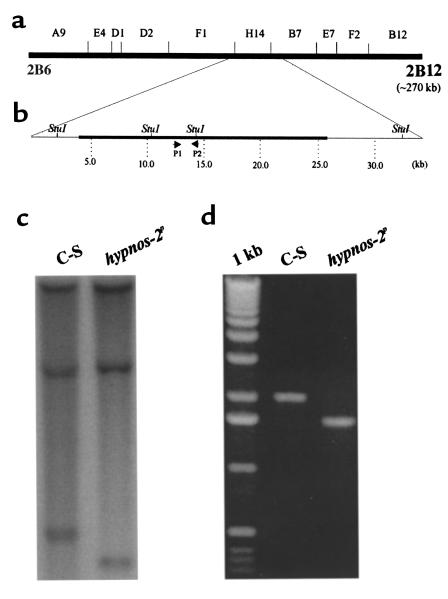
To clone the hypnos-2P gene, Southern blot analysis was performed. Briefly, genomic DNAs from C-S and hypnos-2P flies were isolated and digested with different restriction enzymes (BglII, EcoRI, EcoRV, HindIII, BamHI, KpnI, NdeI, NheI, PstI, PvuII, SacI, ScaI, SphI, StuI, XbaI, XhoI, and XmnI). The digested genomic DNAs were separated in a 1% agarose gel and transferred onto Nytran membranes (Schleicher & Schuell Inc., Keene, New Hampshire, USA). The membranes were separately probed with 10 P32-labeled cosmids purchased from the European Drosophila Genomic Project (EDGP; Greece). These cosmids contained fly genomic DNA fragments that cover a 270-kb region spanning from 2B6 to 2B12, where the mutation of the hypnos-2P was located.
PCR and the targets of hypnos-2 gene.
To confirm the Southern blot analysis results from probe H14 (one of the labeled cosmids; see Results), PCR was performed in the presence of hypnos-2P or C-S genomic DNA and a pair of primers designed according to the cosmid H14 genomic sequence (GenBank accession number AL035207, from 6660 to 6693 and 8670 to 8693).
Since the hypnos-2 gene encodes a pre-mRNA adenosine deaminase that can edit several target molecules, some of these targets were examined in order to find the potential editing “hot spots” in these molecules. The target molecules studied in this work were sodium (para), potassium (shaker), calcium (Dmca1A), and chloride (DrosGluCl-α) channels, and the receptors for glutamate (dGluRI, dGluRIIA, and dGluRIIB) and serotonin. To determine the location of editing sites of these target molecules and the editing ratio at each editing site, we used RT-PCR in the presence of either hypnos-2P or C-S cDNA. Primers used in RT-PCRs are summarized in Table 1. For these PCR reactions, poly (A)+ RNA purified from hypnos-2P and C-S flies was first digested with RNase-free DNase (Boehringer Mannheim Biochemicals Inc., Indianapolis, Indiana, USA) before performing reverse transcription. The PCR products amplified from hypnos-2P or C-S cDNA were cloned into TOPO TA cloning vector (Invitrogen Corp., San Diego, California, USA). For comparison, at least ten clones generated from two sets of PCR reactions were sequenced and analyzed using the LASERGENE program (DNASTAR Inc., Madison, Wisconsin, USA).
Table 1.
Primer sequences used in RT-PCRs for studying site-specific editing of dADAR-target molecules
To examine whether the hypnos-2 gene is expressed in early developmental stages (6- to 8-hour-old embryo) and to determine whether full-length hypnos-2 mRNA is transcribed in hypnos-2P flies, RT-PCR was performed. In the RT-PCR, two primers flanking the deletion region were used (AL035207; forward primer, 5799–5820; reverse primer, 7715–7738).
Transgenic and rescue experiments.
To produce transgenic flies, the whole open reading frame of the hypnos-2 cDNA was subcloned into a PUAST vector. The recombinant construct was purified with a QIAGEN kit (QIAGEN Inc., Valencia, California, USA) and microinjected together with helper DNA (transposase Δ2-3) into 30-minute-old w1118 embryos at a concentration of 1 μg/μl. For rescue experiments, two transgenic fly lines carrying the transgene on the second chromosome (balanced with CyO), as well as two balancers on the third chromosome (TM3 sb and TM6B Hu) were separately crossed with a fly line carrying 32B-GAL4 on the third chromosome (balanced with TM3 sb), as well as two balancers on the second chromosome (Adv and CyO). The male progeny (+/Y; hypnos-2/CyO; 32B/TM3 sb) from these crosses were used to mate hypnos-2P virgin females. The male progeny with a genotype of hypnos-2P/Y; hypnos-2/+; 32B/+ were collected and subjected to an anoxia test at the age of 3–4 days. As controls, male flies with a genotype of +/Y; +/+; 32B/+ or hypnos-2P/Y; +/+; 32B/+ of similar age were tested. In addition, flies of genotype w1118/Y; +/+; +/+ were tested and served as additional controls since w1118 flies were used for the transgenic transformation.
Tissue in situ hybridization.
To examine the distribution of hypnos-2 transcript in the fly head, in situ hybridization was performed as described elsewhere (9). Briefly, 10 μm cryosections from OTC-embedded adult fly heads were probed overnight with S35-labeled antisense or sense RNA probe (∼1.0 × 106 cpm/section). After hybridization, slides were coated with LM-1 emulsion (Amersham Pharmacia Biotech, Piscataway, New Jersey, USA) and incubated for 10 days at 4°C in a light-tight box, developed in Kodak D19 (Eastman Kodak Co., New York, New York, USA), and analyzed using light microscopy.
Drosophila histology.
The freshly dissected fly heads (3 days and 35 days old) were immediately fixed with glutaraldehyde and osmic acid (OsO4). After dehydration, the heads were embedded with durcapan resin (Ted Pella Inc., Redding, California, USA). Serial frontal sections (2.1 μm) were obtained and examined with light microscopy. Four fly heads from each group were sectioned.
Neuronal culture and electrophysiologic recordings.
For our electrophysiologic studies, Drosophila neurons were prepared using O’Dowd’s method (11). Briefly, 5-hour-old embryos from C-S and hypnos-2P mutant flies were collected and bleached. Embryos were then opened, cells dispersed in Drosophila-defined medium 1 (DDM1), and egg shells removed. The dispersed cells were kept in DDM1 in an incubator supplied with 5% CO2 at 25°C up to 14 days. About 90% of differentiated cells prepared in this way were neurons (11). To identify neurons in culture, we used morphologic criteria such as the presence of processes with a pyramidal- or elliptical-shaped soma as described previously (12).
All recordings in Drosophila neurons were performed at room temperature (22–24°C) after 5–10 days in culture. This time period was chosen in order to study cells that have differentiated in culture. Membrane potential was recorded with 0 holding current in the current-clamp mode. Current magnitudes were obtained from current traces in response to either a slow ramp voltage from –160 to –100 mV in the voltage-clamp mode or from voltage-clamp experiments using a command voltage of –20 mV from a holding voltage of –130 mV. Current density was derived from the peak current divided by the whole cell capacitance. For the current-clamp experiments, the external solution contained (in mM/l): 130 NaCl, 3 KCl, 1 CaCl2, 1 MgCl2, 10 HEPES, 10 glucose with pH adjusted to 7.4 with NaOH. The internal pipette solution contained (in mM/l): 138 KCl, 0.2 CaCl2, 1 MgCl2, 10 HEPES (Na+), 10 EGTA with pH adjusted to 7.4 with Tris. The external solutions for the voltage-clamp experiments contained the same salts as in the current-clamp experiments except for 10 mM tetraethyl ammonium (TEA), 5 mM 4-AP, 0.1 mM CdCl2 that replaced equimolar concentrations of NaCl. The internal pipette solution for the voltage-clamp experiments was also similar to that for the current-clamp experiments except for CsCl or CsF substituting for KCl. Cells were used if they had a smooth surface and a three-dimensional contour and were not considered for recording when they had flat or grainy surfaces. Similar criteria have been used by us in the past (12). Electrophysiologic criteria related to seal resistance, holding current magnitude, and series resistance were also used for accepting cells in our analysis (12).
Results
Increased sensitivity of hypnos-2P to lack of O2.
The latency to recovery from stupor induced by a 5-minute period of anoxia is very prolonged in hypnos-2P flies, with a mean recovery time that is more than double that of wild-type C-S flies (615.8 ± 23.5 vs. 321.1 ± 7 seconds; Figure 1a). This increased sensitivity to lack of O2 is also manifested at the time of anoxia induction, as this mutant loses motor control at a significantly faster rate than wild-type flies. We have also observed that the locomotor ability of hypnos-2P flies is limited, although they can jump and move around in the culture vial.
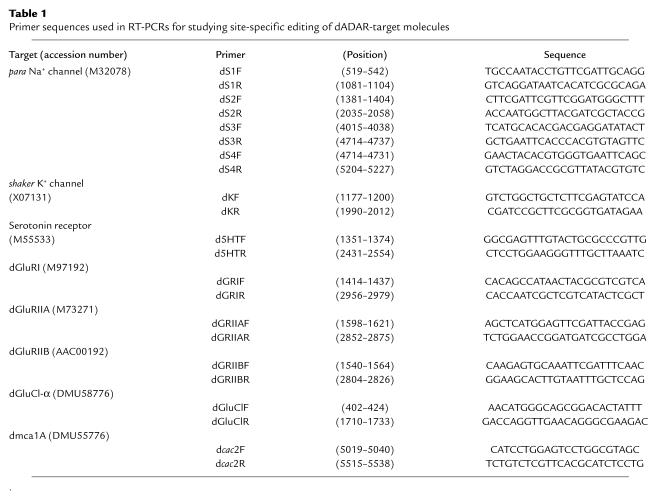
Figure 1.
Complementation test for genetic mapping of hypnos-2P. The hypnos-2P mutation was located previously between genetic markers y (1B1) and cv (5B) on the X chromosome (10). To further map the cytological location of hypnos-2P mutation, genetic crosses between hypnos-2P flies and other deficiencies in this region were carried out (a). The recovery time after 5-minute anoxia was significantly prolonged in the hypnos-2P–mutant flies when compared with either its heterozygotes (hypnos-2P/+) or control (C-S) flies (P < 0.0001). The average recovery time (mean ± SE) for hypnos-2P, heterozygote (hypnos-2P/+), and C-S is 615.8 ± 23.5 seconds (n = 58), 321.1 ± 7 seconds (n = 51), and 324 ± 5.8 seconds (n = 204), respectively. Since the heterozygote female flies (hypnos-2P/+) have a distribution similar to that of the wild-type flies, this mutation is recessive. It should be noted that there are two alleles at the hypnos-2 locus (hypnos-2L and hypnos-2P), but we have focused on hypnos-2P in this work. Our studies showed that hypnos-2P flies failed to complement with the deletions of either df(1)A94 (1E3-2B15) or df(1)st472 (2B6-2B12), but they complemented with df(1)S39 (1E1-2B5). AP < 0.0001. (b) Schematic presentation of cytological locations of three deficiencies used in the complementation tests. The results from these complementation tests indicate that the hypnos-2P mutation is located in the region between 2B6 and 2B12 on the X chromosome.
Other aspects of the hypnos-2P–mutant phenotype.
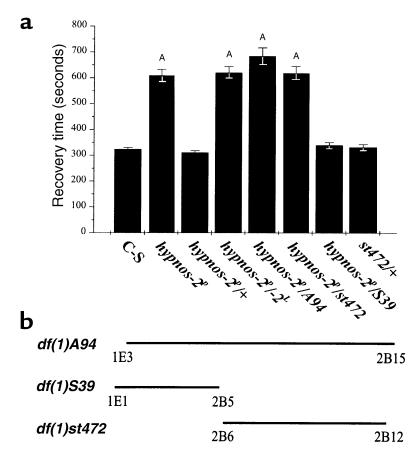
To test whether the mutation of hypnos-2 gene can affect other physiological processes besides the recovery from anoxia, we measured O2 consumption in normal (room air) and low-O2 environment in wild-type and hypnos-2P flies. O2 consumption in the mutants was similar to that in C-S flies in both room air (20.8% of O2) and during low-O2 exposure (1–2% inspired O2). To stress further the metabolic O2 demands in the mutant flies and elicit potential differences between the wild-type and the mutant, we examined the effect of heat shock on both groups of flies. These experiments demonstrated that hypnos-2P–mutant flies were significantly more sensitive to heat shock as compared with wild-type C-S flies. After 2.5 hours at 37°C, adult hypnos-2P flies died at a much higher rate than C-S flies (Figure 2). Moreover, we found that at 25°C the hypnos-2P flies have a shorter life span than C-S flies. For example, approximately 50% of the mutant flies, but only about 15% of C-S flies, had died before they reached 35 days of age. From these data, we conclude that hypnos-2P mutants have an enhanced sensitivity to O2 deprivation, an increased susceptibility to heat shock (which may be related to increased O2 demands or to heat shock itself), and a shorter life span.
Figure 2.
Effect of heat shock on lethality. Heat shock at 37°C has profound effects on hypnos-2P flies, and these die at a much higher proportion than C-S flies (P < 0.05 at 2.5 hours; P < 0.01 at 3 hours; P < 0.0001 at 3.5 to 6 hours). The duration of heat shock that induced 50% death in hypnos-2P flies is significantly shorter than that in C-S flies (3.15 vs. 4.26 hours).
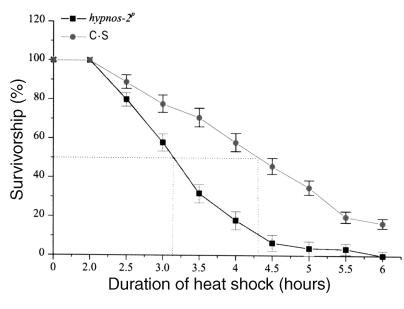
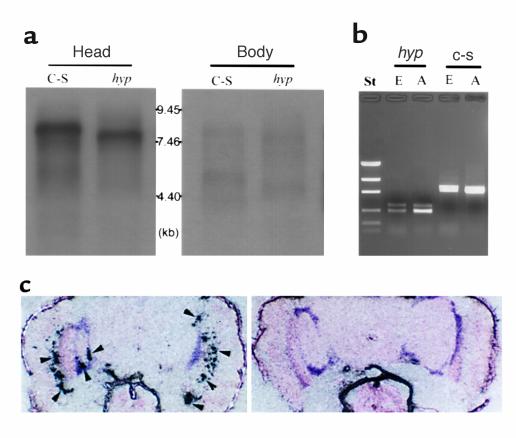
To determine whether the behavioral and physiologic abnormalities are associated with structural alterations in the fly central nervous system (CNS), we examined sections from C-S and mutant brains using light microscopy. Frontal sections (2.1 μm) through adult heads of male flies at 35 days of age clearly showed degeneration in cortical neurons located in the medulla and lobula complex as well as in the lamina of hypnos-2P mutants (Figure 3b, arrowheads), but not in the neurons of control flies. The data of neuronal degeneration in the aged mutant flies are consistent with our findings that the mutant flies have a shorter life span. It should be pointed out that these abnormalities were not seen in young flies (3 days old) from both hypnos-2P and C-S.
Figure 3.
Neuronal degeneration in aged hypnos-2P flies. Frontal sections (2.1 μm) were obtained from durcapan resin–embedded 35-day-old male heads. Neuronal degeneration was detectable in the cortical neurons of medulla and lobula complex as well as in the lamina in hypnos-2P (b) (arrowheads), but not in C-S flies of the same age (a). It should be pointed out that no lesions were observed in 3-day-old adult flies of either hypnos-2P or C-S (data not shown).
Genetic and cytological mapping of hypnos-2P.
Our initial genetic studies have localized hypnos-2P in the region between 1B5 and 5B on the X chromosome (10). After carrying out a series of backcrosses to remove possible mutations at other loci, we performed a series of complementation tests using deficiencies in this region to refine the cytological location of hypnos-2P mutation (Figure 1b). From these complementation tests, we concluded that hypnos-2P mutation is located in the region between 2B6 and 2B12 (Figure 1, a and b). This mutation is a strong amorphic allele because the trans-heterozygote females (hypnos-2P/A94 or hypnos-2P/st472) carrying hypnos-2P mutation over deficiency show a distribution of recovery time similar to that found in hypnos-2P homozygous females (Figure 1a).
Cloning and characterization of hypnos-2 gene.
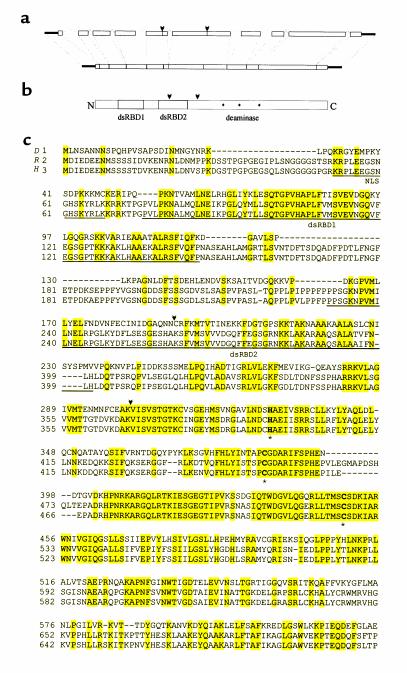
To clone the gene responsible for the hypnos-2P mutation, a series of Southern blot analyses was performed using ten cosmids (purchased from EDGP). These cosmids cover a 270-kb region spanning the whole region of interest (2B6-2B12; Figure 4a). Results from this analysis demonstrated that the mutation of hypnos-2 gene is located in the region covered by the cosmid H14 (Figure 4, b and c). This finding was then confirmed by PCR (Figure 4d). The PCR products were cloned and sequenced. Comparison of the sequenced genomic DNAs from the mutant and wild-type flies revealed that a genomic fragment of 434 bp in hypnos-2P flies was deleted (Figure 5a). Analyzing Drosophila genomic sequence submitted by EDGP showed that this deletion is located in a gene called double-stranded pre-mRNA–specific adenosine deaminase. Since deduced protein of hypnos-2P is highly homologous to the mammalian pre-mRNA adenosine deaminase (ADAR2) (13–17), we also named the hypnos-2 gene dADAR. While this work was in progress, Reenan’s group reported on this Drosophila gene elsewhere (18–20). Sequence analysis showed that two expressed sequence tag (EST) clones contained full-length open reading frames. Comparison between the full-length cDNA and genomic sequence in the fly database showed that the dADAR gene consisted of ten exons and nine introns. The open reading frame of this gene encodes a 632-amino acid protein (632-aa protein) with a molecular weight of 70 kDa (Figure 5, b and c). Like its vertebrate homologues, the dADAR protein contains a typical nuclear localization signal, two double-stranded RNA-binding domains (dsRBDs), and a deaminase domain with three conserved Zn2+-binding residues (Figure 5c). The deletion in the deduced hypnos-2P protein was an in-frame one, and it eliminated the second dsRBD and part of the deaminase domain that is located in exons 5 and 6 (Figure 5, b and c).
Figure 4.
Mapping hypnos-2P mutation. (a) Schematic representation of the cytological location of each of the genomic probes used in the Southern blot analysis. These probes were labeled cosmids and cover the cytological region from 2B6 to 2B12 (∼270 kb) where hypnos-2P mutation is located. From these probes, only probe H14 gave a different hybridization pattern between C-S and hypnos-2P flies. (b) An enlargement of a region covered by probe H14 and both of its flanking ends. (c) A Southern blot analysis showing a deletion in the hypnos-2P flies is located in the region between two StuI sites as indicated in b. (d) PCR analysis confirmed the results from the Southern blot analysis. The PCR products were obtained using the primers shown in b.
Figure 5.
Cloning of a Drosophila pre-mRNA adenosine deaminase (dADAR) (a) Schematic representation of the organization of dADAR gene. The wild-type dADAR gene consists of ten exons and nine introns. In hypnos-2P, 434 bp of genomic DNA, marked by two arrowheads, have been deleted. (b) A domain map shows schematically a deduced dADAR protein that contains two double-stranded RNA-binding domains (dsRBDs; shaded boxes) and the deaminase domain with its putative Zn+-chelating residues (asterisks). (c) Alignment among Drosophila (D), rat (R; accession number S78526), and human (H; accession number X99383) ADAR proteins. Gaps are introduced for optimal alignment; identical residues are highlighted. The nuclear localization signal (NLS) is underlined and labeled at the end of the NH2 terminal. The two dsRBDs are underlined and labeled, and the three Zn+-chelating residues are bold and marked with asterisks. The deleted fragment in hypnos-2P mutation is marked by two flanking arrowheads. The identities between dADAR and rADAR and between dADAR and hADAR are 43% and 44%, respectively. The GenBank accession number for dADAR cDNA is AF343579.
Wild-type dADAR transgene rescues the resistance to O2 deprivation of hypnos-2P flies.
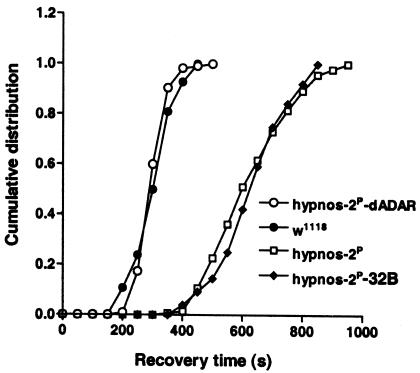
To ensure that the phenotype displayed in hypnos-2P flies is not due to the perturbation of neighboring genes that may be indirectly disrupted by the genetic lesion and to provide definitive evidence that the mutation of dADAR gene is responsible for the sensitivity to anoxia shown in hypnos-2P flies, a transformation rescue experiment with a wild-type dADAR transgene was performed. All aspects of the phenotype that appeared in hypnos-2P flies were rescued by the transgene. Hypnos-2P male flies carrying the wild-type UAS transgene driven by 32B-GAL4 (hypnos-2P-dADAR) show no prolongation in recovery time from a 5-minute anoxia when compared with control male flies (w1118) (317.4 ± 4.2 seconds vs. 314.2 ± 7.2 seconds, respectively; Figure 6). Furthermore, uncoordinated locomotion was also rescued by the transgene. Data from our complementation tests, rescue experiments, and studies on dADAR targets (see below) demonstrate, therefore, that removal of the second dsRBD and part of the deaminase domain eliminates entirely the editing activity of dADAR.
Figure 6.
Transformation rescue. Since the deletion in the dADAR gene is located on the X chromosome, only male flies were tested, and the recovery from a 5-minute anoxia was presented as cumulative frequency. Male hypnos-2P-32B and w1118 flies served as controls. The prolonged recovery of hypnos-2P flies can be fully rescued by a wild-type dADAR transgene. The average recovery time (mean ± SE) for hypnos-2P male flies carrying the wild-type UAS transgene driven by 32B-GAL4 is similar to that for w1118 males (hypnos-2P-dADAR vs. w1118: 317.4 ± 4.2 seconds, n = 115, vs. 314.2 ± 7.2 seconds, n = 84). Note also that almost 100% of flies from both w1118 and hypnos-2P-dADAR groups had recovered before any fly recovered from the hypnos-2P group. As controls, hypnos-2P male flies and male flies carrying hypnos-2P and promoter 32B-GAL4 without the dADAR transgene (hypnos-2P-32B) had a similar recovery time (638.8 ± 11.4 seconds, n = 141, vs. 646.8 ± 13.6 seconds, n = 76, respectively).
Expression of dADAR gene.
To study the tissue distribution and the developmental profile of expression of this gene, nucleic acid hybridization and PCR were performed (9). Northern blot analysis showed that a major transcript band at approximately 8.0 kb existed mainly in the adult head and the transcripts in hypnos-2P were smaller than those in C-S (Figure 7a), indicating that the deletion in hypnos-2P is an in-frame one, as was shown by our direct sequence. Furthermore, the expression of this gene is developmentally regulated. A band of 3.5 kb was found only in 1- to 3-hour-old embryos. From late embryonic to early pupal stages, this gene is expressed at a low level (data not shown). To confirm the results from the Northern blot analysis and to prove that hypnos-2P does not express full-length dADAR transcripts, RT-PCR was performed. The results from these PCR experiments showed that dADAR mRNA is detectable in young embryos (for example, 6–8 hours) and that no full-length of dADAR transcripts was observed in both hypnos-2P embryo and adult (Figure 7b). We also found that there are several alternative splicing isoforms of dADAR, and these isoforms are developmentally regulated.
Figure 7.
Expression of dADAR gene. (a) Northern blot analysis. Poly (A)+ RNA is isolated using a FastTrack kit (Invitrogen Corp.). Ten micrograms of poly (A)+ RNA from each group are fractionated by electrophoresis on a 1.2% agarose gel (FMC Corp., Chicago, Illinois, USA) containing formaldehyde and hybridized with P32-labeled dADAR cDNA probe. The blot hybridization shows that dADAR transcripts are expressed mainly in adult heads, and the deletion in hypnos-2P is an in-frame deletion. (b) RT-PCR. The expression of dADAR can be detected by RT-PCR in C-S embryos (6–8 hours), although it is hardly detectable by Northern blot analysis (data not shown). It should be noted that no full-length dADAR transcripts can be detected in hypnos-2P embryos and adults. St, standard. (c) Localization of dADAR transcripts in adult fly heads. An intense signal was observed in neurons of the lamina and cortical regions of central brain (left panel; arrowheads). Sense dADAR RNA probe gave no positive signals in the same tissues (right panel). The DNA marker in b is low-mass DNA standard. hyp, hypnos-2P; E, embryos (6–8 hours); A, adults.
To study the distribution of dADAR transcripts in the fly head, in situ hybridization with dADAR cRNA probe was carried out. An intense signal was observed only in neurons of lamina and the cortical region of central brain (Figure 7c, arrowheads). Hence, the results from both Northern and in situ hybridization analyses reveal that the dADAR gene is expressed primarily in the adult brain. The neuronal-specific expression of dADAR mRNA in the CNS of the adult fly supports our previous electrophysiologic Giant Fiber data on the mutants. We demonstrated that the mutant flies have either a total lack of evoked potentials (EP) or a marked delay of EP for recovery from anoxia when Giant neurons were stimulated (10).
Target molecules of dADAR.
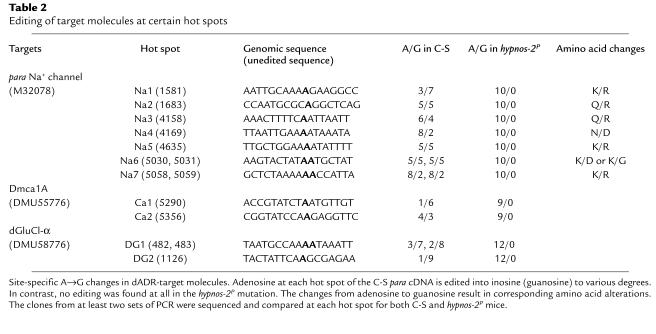
It has become clear that pre-mRNA editing by ADAR plays an important role in a number of biological processes. For example, it has been reported that ADAR can edit several substrates such as the pre-mRNAs for glutamate and serotonin receptors (21–24), voltage-gated channels for K+, Na+, and Ca2+ (18, 20, 25, 26), and the glutamate-gated Cl– channel (DrosGluCl-α) (27). To analyze the editing activities of dADAR on these targets in both C-S and hypnos-2P mutants, we performed RT-PCR, and their products were cloned and sequenced. The results from these experiments show that dADAR extensively edits Na+ (para), Ca2+ (Dmca1A), and chloride (DrosGluCl-α) ion channel mRNAs (Table 2). For example, the Drosophila para Na+ channel is edited at seven hot spots (Figure 8, a and b) and results in eight amino acid changes in the coding region. In wild-type Na+-channel transcripts, the editing ratio varies from one hot spot to another, ranging from 20 to 70% (Table 2). The editing activity was totally eliminated in hypnos-2P flies, indicating that the dADAR activity was totally abolished in these flies (Table 2). Our studies showed also that, unlike mammals (15, 17, 21, 22), shaker-like K+ channel and receptors for glutamate and serotonin are not edited in the wild-type fruit fly.
Table 2.
Editing of target molecules at certain hot spots
Figure 8.
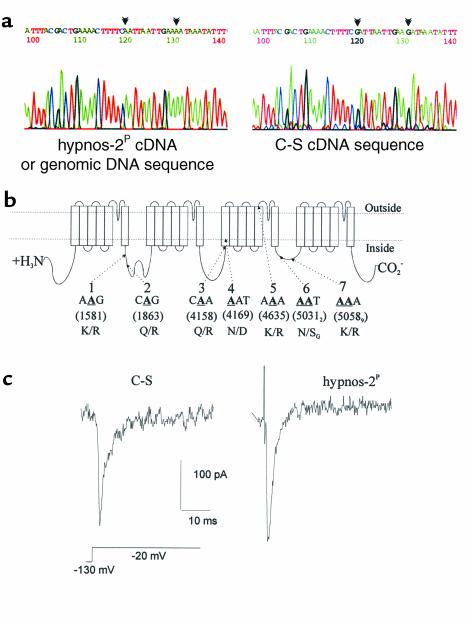
Na+-channel editing and neuronal electrophysiological properties. (a) An electrophoregram showing the sequences of hot spots 3 and 4 (described in b). The left panel shows the hypnos-2P cDNA sequence surrounding these two spots. No editing was seen in the mutant since its cDNA sequence is the same as its genomic sequence; the right panel represents the edited cDNA sequence in wild-type C-S flies. Editing sites are marked by arrowheads. (b) Schematic presentation of the para sodium channel showing hot spots that are edited in its pre-mRNA form. From PCR analyses we found that at least seven hot spots (or nine editing sites) in this Na+ channel pre-mRNA are edited by the dADAR. Potentially edited adenosines (labeled A) are shown in bold form, underlined, and numbered in parentheses according to the para Na+-channel cDNA sequence (GenBank accession number M32078). (c) Recording of neuronal sodium current using whole-cell voltage clamp. Ca2+ and K+ currents were blocked using cadmium, 4-AP and TEA (extracellular), and cesium (intracellular; see Methods). This is an example of a Na+-channel current from hypnos-2P and C-S. Note that a larger Na+ current was seen in hypnos-2P as compared with the wild-type C-S.
As an initial attempt to determine whether the lack of editing in the channel transcripts can affect their functions, we cultured neurons from wild-type and hypnos-2P–mutant embryos, performed current and voltage-clamp recordings, and focused on one of the channels, namely the Na+ channel (Figure 8c). About 30–40% of both mutant and C-S neurons from at least six different cultures expressed Na+ currents, and most of these neurons fired action potentials. The membrane potential in the mutant was significantly lower than in wild-type neurons (–41 ± 3.2 mV, n = 29 in C-S, and –32 ± 6 mV, n = 18 in the mutant; P < 0.05). It is interesting, however, that despite a less-negative membrane potential in the mutant cells, the Na+-channel current density, or peak Na+-current normalized to capacitance, was significantly higher in the mutant than in C-S neurons (120 ± 43 pA/pF, n = 11, vs. 91 ± 18 pA/pF, n = 22; P < 0.05, Wilcoxon rank sum test). These results provide the first in vivo evidence that there are functional electrophysiological consequences resulting from the lack of editing of the Na+ channel by the adenosine deaminase.
Discussion
We have shown in this work that the mutation of dADAR in hypnos-2P significantly prolonged the recovery from anoxic stupor and led to neuronal degeneration in aged flies. All aspects of the hypnos-2P phenotype resulted from the partial deletion of the dADAR gene since these can be fully rescued by a wild-type dADAR transgene. Although we cannot rule out the existence of other targets for this deaminase, it is highly likely that the extensive editing of Na+, Ca2+, and chloride channels found in this study and others (26, 27) plays an important role in this anoxia tolerance and in the phenotype described. The importance of the integrity of genes encoding for channels, such as the voltage-gated para Na+ and Dmca1A channels, have been shown using genetic and molecular techniques to be essential in the maintenance of normal neuronal functions. For example, disruption of either of the para Na+ or the cac Ca2+ channel gene will generate abnormal neurophysiological and behavioral phenotypes (28, 29) and disruption of A→G RNA editing can result in severe neurologic dysfunction (19–22). Hence, the activity of membrane proteins such as certain voltage-gated channels are highly controlled by the adenosine deaminase, which is regulated not only during development but also in a tissue-specific manner, as we showed in this work. Of relevance to our current data are in vitro electrophysiologic studies that have shown that these membrane proteins, especially the voltage-gated Na+ and Ca2+ channels, play a pivotal role in nerve cell injury caused by O2 deprivation (1, 30–32). Therefore, taken together, these data lead us to hypothesize that the phenotypic consequences of hypnos-2P mutation reported here are, at least in part, due to the absence of editing of pre-mRNAs for these Drosophila ion channels in the mutant flies. This idea is supported by our electrophysiologic recordings showing that the lack of editing of para Na+ in hypnos-2P–mutant neurons can lead to an increase in Na+ influx into neurons, especially during low O2 states, an important phenomenon that we and others have studied in mammalian tissues (30, 32). These channel proteins in their unedited pre-mRNA form (resulting from a defective adenosine deaminase) may have different functional properties that disturbs neuronal ionic homeostasis and leads to prolonged recovery from anoxic stupor, decreased survival during high O2 demands or heat shock, and neuronal degeneration in aged mutant flies. Indeed, an increase in Na+ influx into neurons can result in severe neuronal injury (30). This gain of function in Na+ channel current is similar to that of editing-deficient glutamate-receptor subtype IIB, which resulted in an increase in Ca2+ and Na+ influx, severe neurologic dysfunction, and early-onset epilepsy (21, 22). Given the biological importance and phylogenetic conservation of ADAR, cloning and characterization of the dADAR will facilitate our understanding of genetic and molecular bases for the tolerance to lack of O2 and environmental stress in mammals and humans.
Acknowledgments
This study was supported by grants from the American Heart Association (0030065N), March of Dimes (6-FY00-99), and NIH (NS37756-01A1). We thank Pietro De Camilli for reading this manuscript and for his suggestions, Igor Zhimulev for supplying st472 mutant flies, and Kendra Swirsding and Xiaolan Fei for technical assistance.
References
- 1.Choi DW, Rothman SM. The role of glutamate neurotoxicity in hypoxic-ischemic neuronal death. Annu Rev Neurosci. 1990;13:171–182. doi: 10.1146/annurev.ne.13.030190.001131. [DOI] [PubMed] [Google Scholar]
- 2.Kuwabara K, et al. Purification and characterization of a novel stress protein, the 150-kDa oxygen-regulated protein (ORP150), from cultured rat astrocytes and its expression in ischemic mouse brain. J Biol Chem. 1996;271:5025–5032. doi: 10.1074/jbc.271.9.5025. [DOI] [PubMed] [Google Scholar]
- 3.Carmeliet P, et al. Role of HIF-1alpha in hypoxia-mediated apoptosis, cell proliferation and tumour angiogenesis [erratum 1998, 395:525] Nature. 1998;394:485–490. doi: 10.1038/28867. [DOI] [PubMed] [Google Scholar]
- 4.Dachs GU, et al. Targeting gene expression to hypoxic tumor cells. Nat Med. 1997;3:515–520. doi: 10.1038/nm0597-515. [DOI] [PubMed] [Google Scholar]
- 5.Artavanis-Tsakonas S, Matsuno K, Fortini ME. Notch signaling. Science. 1995;268:225–232. doi: 10.1126/science.7716513. [DOI] [PubMed] [Google Scholar]
- 6.Jan LY, Jan YN. Tracing the roots of ion channels. Cell. 1992;69:715–718. doi: 10.1016/0092-8674(92)90280-p. [DOI] [PubMed] [Google Scholar]
- 7.Rauskolb C, Peifer M, Wieschaus E. Extradenticle, a regulator of homeotic gene activity, is a homolog of the homeobox-containing human proto-oncogene pbx1. Cell. 1993;74:1101–1112. doi: 10.1016/0092-8674(93)90731-5. [DOI] [PubMed] [Google Scholar]
- 8.Ma E, Haddad GG. Anoxia regulates gene expression in the central nervous system of Drosophila melanogaster. Brain Res Mol Brain Res. 1997;46:325–328. doi: 10.1016/s0169-328x(97)00074-0. [DOI] [PubMed] [Google Scholar]
- 9.Ma E, Xu T, Haddad GG. Gene regulation by O2 deprivation: an anoxia-regulated novel gene in Drosophila melanogaster. Brain Res Mol Brain Res. 1999;63:217–224. doi: 10.1016/s0169-328x(98)00265-4. [DOI] [PubMed] [Google Scholar]
- 10.Haddad GG, Sun Y, Wyman RJ, Xu T. Genetic basis of tolerance to O2 deprivation in Drosophila melanogaster. Proc Natl Acad Sci USA. 1997;94:10809–10812. doi: 10.1073/pnas.94.20.10809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O’Dowd DK. Voltage-gated currents and firing properties of embryonic Drosophila neurons grown in a chemically defined medium. J Neurobiol. 1995;27:113–126. doi: 10.1002/neu.480270111. [DOI] [PubMed] [Google Scholar]
- 12.Gu XQ, Haddad GG. Drosophila neurons respond differently to hypoxia and cyanide than rat neurons. Brain Res. 1999;845:6–13. doi: 10.1016/s0006-8993(99)01877-6. [DOI] [PubMed] [Google Scholar]
- 13.Bass BL, et al. A standardized nomenclature for adenosine deaminases that act on RNA. RNA. 1997;3:947–949. [PMC free article] [PubMed] [Google Scholar]
- 14.Melcher T, et al. A mammalian RNA editing enzyme. Nature. 1996;379:460–464. doi: 10.1038/379460a0. [DOI] [PubMed] [Google Scholar]
- 15.Melcher T, et al. RED2, a brain-specific member of the RNA-specific adenosine deaminase family. J Biol Chem. 1996;271:31795–31798. doi: 10.1074/jbc.271.50.31795. [DOI] [PubMed] [Google Scholar]
- 16.Mittaz L, et al. Cloning of a human RNA editing deaminase (ADARB1) of glutamate receptors that maps to chromosome 21q22.3. Genomics. 1997;41:210–217. doi: 10.1006/geno.1997.4655. [DOI] [PubMed] [Google Scholar]
- 17.Lai F, Chen CX, Carter KC, Nishikura K. Editing of glutamate receptor B subunit ion channel RNAs by four alternatively spliced DRADA2 double-stranded RNA adenosine deaminases. Mol Cell Biol. 1997;17:2413–2424. doi: 10.1128/mcb.17.5.2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hanrahan CJ, Palladino MJ, Ganetzky B, Reenan RA. RNA editing of the Drosophila para Na(+) channel transcript. Evolutionary conservation and developmental regulation. Genetics. 2000;155:1149–1160. doi: 10.1093/genetics/155.3.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reenan RA, Hanrahan CJ, Barry G. The mle(napts) RNA helicase mutation in Drosophila results in a splicing catastrophe of the para Na+ channel transcript in a region of RNA editing. Neuron. 2000;25:139–149. doi: 10.1016/s0896-6273(00)80878-8. [DOI] [PubMed] [Google Scholar]
- 20.Palladino MJ, Keegan LP, O’Connell MA, Reenan RA. A-to-I pre-mRNA editing in Drosophila is primarily involved in adult nervous system function and integrity. Cell. 2000;102:437–449. doi: 10.1016/s0092-8674(00)00049-0. [DOI] [PubMed] [Google Scholar]
- 21.Brusa R, et al. Early-onset epilepsy and postnatal lethality associated with an editing-deficient GluR-B allele in mice. Science. 1995;270:1677–1680. doi: 10.1126/science.270.5242.1677. [DOI] [PubMed] [Google Scholar]
- 22.Higuchi M, et al. Point mutation in an AMPA receptor gene rescues lethality in mice deficient in the RNA-editing enzyme ADAR2. Nature. 2000;406:78–81. doi: 10.1038/35017558. [DOI] [PubMed] [Google Scholar]
- 23.Sommer B, Kohler M, Sprengel R, Seeburg PH. RNA editing in brain controls a determinant of ion flow in glutamate-gated channels. Cell. 1991;67:11–19. doi: 10.1016/0092-8674(91)90568-j. [DOI] [PubMed] [Google Scholar]
- 24.Burns CM, et al. Regulation of serotonin-2C receptor G-protein coupling by RNA editing. Nature. 1997;387:303–308. doi: 10.1038/387303a0. [DOI] [PubMed] [Google Scholar]
- 25.Patton DE, Silva T, Bezanilla F. RNA editing generates a diverse array of transcripts encoding squid Kv2 K+ channels with altered functional properties. Neuron. 1997;19:711–722. doi: 10.1016/s0896-6273(00)80383-9. [DOI] [PubMed] [Google Scholar]
- 26.Smith LA, Peixoto AA, Hall JC. RNA editing in the Drosophila DMCA1A calcium-channel alpha 1 subunit transcript. J Neurogenet. 1998;12:227–240. doi: 10.3109/01677069809108560. [DOI] [PubMed] [Google Scholar]
- 27.Semenov EP, Pak WL. Diversification of Drosophila chloride channel gene by multiple posttranscriptional mRNA modifications. J Neurochem. 1999;72:66–72. doi: 10.1046/j.1471-4159.1999.0720066.x. [DOI] [PubMed] [Google Scholar]
- 28.Ganetzky B. Genetic studies of membrane excitability in Drosophia: lethal interaction between two temperature-sensitive paralytic mutations. Genetics. 1984;108:897–911. doi: 10.1093/genetics/108.4.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith LA, et al. A Drosophila calcium a1 subunit gene maps to a genetic locus associated with behavioral and visual defects. J Neurosci. 1996;16:7868–7879. doi: 10.1523/JNEUROSCI.16-24-07868.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vornov JJ. Ion channels and exchangers that mediate ischemic neuronal injury. Curr Opin Neurol. 1998;11:39–43. doi: 10.1097/00019052-199802000-00007. [DOI] [PubMed] [Google Scholar]
- 31.Kimura M, et al. Role of glutamate receptors and voltage-dependent calcium and sodium channels in the extracellular glutamate/aspartate accumulation and subsequent neuronal injury induced by oxygen/glucose deprivation in cultured hippocampal neurons. J Pharmacol Exp Ther. 1998;285:178–185. [PubMed] [Google Scholar]
- 32.Haddad GG, Jiang C. O2-sensing mechanisms in excitable cells: role of plasma membrane K+ channels. Annu Rev Physiol. 1997;59:23–42. doi: 10.1146/annurev.physiol.59.1.23. [DOI] [PubMed] [Google Scholar]