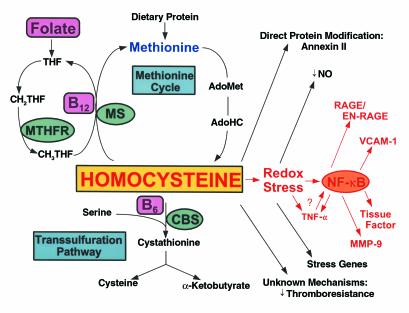
Homocysteine (HC) is a non–protein-forming, sulfur-containing amino acid that stands at the crossroads of two metabolic pathways (Figure 1) (1). HC, which is not a dietary constituent, is formed exclusively upon demethylation of methionine, and is eliminated through one of two vitamin-dependent pathways, in addition to an alternate vitamin-independent pathway confined to the liver. In the ubiquitous, vitamin B12–dependent remethylation pathway, methionine synthase (MS) catalyzes the conversion of HC back to methionine in the presence of the methyl donor, N-5-methyltetrahydrofolate. Alternatively, HC can undergo transsulfuration, in which it condenses with serine to form cystathionine through the action of the vitamin B6–containing enzyme, cystathionine-β-synthase (CBS). Cystathionine is then hydrolyzed to cysteine and α-ketobutyrate by γ-cystathioninase.
Figure 1.
HC metabolism and vascular dysfunction. HC is formed upon demethylation of methionine via S-adenosylmethionine (AdoMet) and S-adenosylhomocysteine (AdoHC). HC is eliminated in the methionine cycle by remethylation through the action of methionine synthase (MS), a vitamin B12–dependent enzyme. In this reaction, methyltetrahydrofolate (CH3THF) serves as the methyl donor, and is formed from methylene tetrahydrofolate (CH2THF) through the action of methyltetrahydrofolate reductase (MTHFR). CH2THF is formed from tetrahydrofolate (THF), a folate derivative. Alternatively, HC can participate in transsulfuration in which it condenses with serine through the action of the vitamin B6-dependent enzyme, cystathionine-β-synthase (CBS). Cystathionine then splits into cysteine and α-ketobutyrate. HC can modify vascular cell function by forming a direct disulfide protein derivative, as in the case of the fibrinolytic receptor, annexin II; by inducing a prothrombotic phenotype through largely unknown mechanisms; or by undergoing auto-oxidation generating superoxide radicals (redox stress), leading to depletion of nitric oxide (NO•) and expression of acute stress–related genes. Redox stress may also activate the proinflammatory transcription factor NF-κB, which may induce expression of TNF-α, RAGE/EN-RAGE, VCAM-1, tissue factor, and MMP-9. TNF-α may also induce activation and nuclear translocation of NF-κB.
Elevated plasma levels of HC have been associated with vascular disease since the initial descriptions of classical homocystinuria in children. Gerritsen and Waisman first reported a fatal pulmonary thromboembolism in a one-year-old with homocystinuria (2). Others found autopsy evidence of thrombosis of the superior sagittal sinus, inferior vena cava, and portal vein, as well as atherosclerosis-like occlusion of renal, carotid, and coronary arteries (3). McCully first described intimal fibrosis and disruption of the internal elastic lamina in multiple medium-sized arteries in an infant with MS deficiency and a child with CBS deficiency (4).
In 1976, Wilcken and Wilcken showed in the JCI that, compared with normal subjects, those with coronary artery disease exhibited higher plasma HC levels following a challenge with oral methionine (5). Indeed, the last decade has witnessed an exponential increase in studies defining plasma HC as an independent risk factor, similar to smoking or hyperlipidemia, for atherosclerotic cardiovascular, cerebrovascular, and peripheral vascular disease, and for deep vein thrombosis and thromboembolism (6, 7). A meta-analysis of over 4,000 patients showed a graded risk for atherosclerosis of cardiovascular, cerebrovascular, and peripheral vessels such that a 5 μM increase in HC conferred a 80% increased risk to women and a 60% increased risk to men (8). A clear association also exists between plasma HC and mortality in both patients with angiographic coronary artery disease (9) and the elderly (10).
The first in vivo evidence that HC can alter endothelial-cell function in the absence of pre-existing vascular disease was published in the JCI in 1996 (11). Healthy cynomolgus monkeys made mildly hyperhomocysteinemic by a high methionine, low folate diet showed markedly impaired responses to endothelium-dependent vasodilators such as acetylcholine and ADP. Eberhardt et al. reinforced this view in a second study in the JCI involving mice heterozygous for the CBS null allele (12). These animals, which maintained mild hyperhomocysteinemia in the absence of structural vascular disease (13), showed attenuated endothelium-dependent aortic relaxation to acetylcholine but a normal endothelium-independent response to sodium nitroprusside. Such work has led to the oxidant stress theory of vascular dysfunction, according to which the sulfhydryl group of HC undergoes auto-oxidation, generating superoxide radicals (O2–) which consume the vasodilator NO to form peroxynitrite (OONO–) (14).
In the current issue of the JCI, Hofmann et al. show for the first time that HC can accelerate the development of atherosclerotic lesions in a genetically susceptible animal, and that this process can be inhibited by supplementation with B vitamins and folate (15). ApoE-null mice fed a normal chow diet become hypercholesterolemic, developing foam-cell accumulation after 10 weeks and advanced fibrous plaques after 15 weeks (16). However, when these animals were placed for 8 weeks on a diet enriched in methionine and deficient in folic acid, B6, and B12, they developed atherosclerotic lesions of increased size and complexity, a finding which could not be attributed to low vitamin status.
Atherogenesis and inflammation
The late Russell Ross noted that the earliest sign of atherosclerosis, the fatty streak, is a purely inflammatory lesion that forms in response to arterial injury (17). Indeed, Hofmann et al. (15) demonstrate further that HC unleashes a veritable storm of inflammatory mediators. Unlike ApoE-null controls, mice that are both ApoE-deficient and hyperhomocysteinemic show nuclear translocation of NF-κB, a redox-activated inflammatory transcription factor (18); high plasma levels of the inflammatory cytokine TNF-α; and increased expression of the receptor for advanced glycation end-products (RAGE) and its signal-transducing ligand (EN-RAGE), newly identified signaling partners in chronic inflammation (19). Several downstream gene products, VCAM-1, tissue factor, and matrix metalloproteinase-9 are also activated. Remarkably, none of these effects are observed in mice on a diet replete in HC-lowering vitamins.
While these findings add to the panoply of known HC-induced vascular perturbations, they also raise some additional “burning” questions. In addition to blocking thromboresistance, inhibiting cell surface fibrinolysis, inducing stress-response genes, and impairing endothelium-dependent vasorelaxation (20), HC now appears to initiate a cascade of inflammatory pathways possibly sparked by NF-κB, fueled by RAGE, and resulting in a hyperadhesive, prothrombotic, elastolytic vascular cell. Challenges for the future will be to determine how these pathways are initiated, where they intersect, and whether they are confined to vascular tissues.
In Shakespeare’s King Lear (21), a great storm gathers as he becomes consumed with anger at his daughters’ ingratitude. As Lear descends into madness, he rages against the elements, “Blow winds and crack your cheeks! Rage! Blow! You sulph’rous and thought-executing fires, Singe my white head!”
HC now appears to engender its own brand of sulph’rous fire within the arterial wall. Ongoing clinical trials will soon reveal whether the flame can be extinguished in humans by the simple act of replenishing three dietary vitamins.
References
- 1.Selhub J. Homocysteine metabolism. Ann Rev Nutr. 1999;19:217–246. doi: 10.1146/annurev.nutr.19.1.217. [DOI] [PubMed] [Google Scholar]
- 2.Gerritsen T, Waisman HA. Homocystinuria, an error in the metabolism of methionine. Pediatrics. 1964;33:413–420. [PubMed] [Google Scholar]
- 3.Mudd, H., Levy, H.L., and Skovby, F. 1995. Disorders of transsulfuration. In The metabolic and molecular basis of inherited disease. C.R. Scriver, A.L. Beaudet, W.S. Sly, and D. Valle, editors. McGraw-Hill Inc. New York, New York, USA. 1279–1327.
- 4.McCully KS. Vascular pathology of homocysteinemia: implications for the pathogenesis of arteriosclerosis. Am J Pathol. 1969;56:111–128. [PMC free article] [PubMed] [Google Scholar]
- 5.Wilcken DEL, Wilcken B. The pathogenesis of coronary artery disease. A possible role for methionine metabolism. J Clin Invest. 1976;57:1079–1082. doi: 10.1172/JCI108350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Graham IM, et al. Plasma homocysteine as a risk factor for vascular disease: the European Concerted Action Project. JAMA. 1997;277:1775–1781. doi: 10.1001/jama.1997.03540460039030. [DOI] [PubMed] [Google Scholar]
- 7.D’Angelo A, Selhub J. Homocysteine and thrombotic disease. Blood. 1997;90:1–11. [PubMed] [Google Scholar]
- 8.Boushey CJ, Beresford SAA, Omenn GS, Motulsky AG. A quantitative assessment of plasma homocysteine as a risk factor for vascular disease. JAMA. 1995;274:1049–1057. doi: 10.1001/jama.1995.03530130055028. [DOI] [PubMed] [Google Scholar]
- 9.Nygard O, et al. Plasma homocysteine levels and mortality in patients with coronary artery disease. N Engl J Med. 1997;337:230–236. doi: 10.1056/NEJM199707243370403. [DOI] [PubMed] [Google Scholar]
- 10.Bostom AG, et al. Nonfasting plasma total homocysteine levels and all-cause and cardiovascular disease mortality in elderly Framingham men and women. Arch Intern Med. 1999;159:1077–1080. doi: 10.1001/archinte.159.10.1077. [DOI] [PubMed] [Google Scholar]
- 11.Lentz SR, et al. Vascular dysfunction in monkeys with diet-induced hyperhomocyst(e)inemia. J Clin Invest. 1996;98:24–29. doi: 10.1172/JCI118771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eberhardt RT, et al. Endothelial dysfunction in a murine model of mild hyperhomocyst(e)inemia. J Clin Invest. 2000;106:483–491. doi: 10.1172/JCI8342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Watanabe M, et al. Mice deficient in cystathionine beta-synthase: animal models for mild and severe homocyst(e)inemia. Proc Natl Acad Sci USA. 1995;92:1585–1589. doi: 10.1073/pnas.92.5.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Loscalzo J. The oxidant stress of hyperhomocyst(e)inemia. J Clin Invest. 1996;98:5–7. doi: 10.1172/JCI118776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hofmann MA, et al. Hyperhomocysteinemia enhances vascular inflammation and accelerates atherosclerosis in a murine model. J Clin Invest. 2001;107:675–683. doi: 10.1172/JCI10588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nakashima Y, Plump AS, Raines EW, Breslow JL, Ross RS. ApoE-deficient mice develop lesions of all phases of atherosclerosis throughout the arterial tree. Arterioscler Thromb. 1994;14:133–140. doi: 10.1161/01.atv.14.1.133. [DOI] [PubMed] [Google Scholar]
- 17.Ross R. Atherosclerosis: an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 18.Janssen-Heininger YMW, Poynter ME, Baeuerle PA. Recent advances towards understanding redox mechanisms in the activation of nuclear factor κB. Free Rad Biol Med. 2000;28:1317–1327. doi: 10.1016/s0891-5849(00)00218-5. [DOI] [PubMed] [Google Scholar]
- 19.Hofmann MA, et al. RAGE mediates a novel proinflammatory axis: a central cell surface receptor for S100/calgranulin polypeptides. Cell. 1999;97:889–901. doi: 10.1016/s0092-8674(00)80801-6. [DOI] [PubMed] [Google Scholar]
- 20.Ling Q, Hajjar KA. Inhibition of endothelial cell thromboresistance by homocysteine. J Nutr. 2000;130:373S–376S. doi: 10.1093/jn/130.2.373S. [DOI] [PubMed] [Google Scholar]
- 21.Shakespeare, W. 1606. King Lear. Act III, scene II, lines 1–6.