In this issue of the JCI, Kosiewicz and her colleagues (1) provide valuable clinical data and the first systematic immunologic study of the SAMP1/Yit mouse, a murine model of spontaneous gastrointestinal inflammation that bears remarkable similarity to human Crohn’s disease. The mouse strain used has its origin in strains developed in the early 1980s for a very different purpose. Hoping to study the genetics of aging, Takeda et al. interbred AKR mice to generate senescence-prone or senescence-resistant sublines, including the short-lived strain SAMP1 (2). In the 1990s, Matsumoto et al. established a new subline of the SAMP1 mouse with spontaneous skin ulcerations (3). The SAMP1/Yit strain, which arose from SAMP1, no longer undergoes accelerated senescence, but it exhibits the novel phenotype of spontaneous intestinal inflammation. Genetic analysis in the present report shows that approximately 40% of the polymorphic alleles in the SAMP1/Yit genome differ from the original AKR strain, indicating that genetic material from one or more other strains was introduced into this line at some point in the SAMP1/Yit pedigree. Regardless of the origin of these unexpected alleles, SAMP1/Yit is now an inbred strain of great promise for the study of Crohn’s disease pathogenesis.
As described initially by Matsumoto et al. (3), the intestinal lesions in the SAMP1/Yit mouse strain consist of discontinuous inflammatory lesions occurring mainly in the terminal ileum, and to a lesser extent the cecum, which are more or less similar to those occurring in Crohn’s disease. These lesions consist of an extensive infiltration of CD3+ T cells, macrophages, and neutrophils, which occur in association with epithelial cell hyperplasia, crypt elongation, and crypt microabscesses. Of great interest, Matsumoto et al. also found that, when raised under strictly aseptic (gnotobiotic) conditions, SAMP1/Yit mice remain free of intestinal disease (3). Under more standard conditions, which eliminate pathogenic bacteria but not benign intestinal microflora, these mice invariably develop disease by 15 weeks of age.
Early animal studies on inflammatory bowel disease
Attempts to develop animal models of inflammatory bowel disease (IBD) have a long history, as summarized in a review of early work in this area by Strober in 1985 (4). Animal model studies by Kirsner and his colleagues in the 1950s and 1960s represented some of the first credible research on possible immunologic factors in IBD pathogenesis (5). These and subsequent investigators developed models of intestinal inflammation by first inducing antibodies to an exogenous antigen and then administering the antigen along with formalin per rectum to generate antigen-antibody complexes in the large intestine. The pathologic Auer lesions thus obtained were somewhat like those seen in ulcerative colitis but were quite short-lived; however, they could be made more chronic by using immunizing antigens derived from normal gut constituents, particularly antigens associated with resident Escherichia coli (6). This established the principle quite early that chronic inflammation of the gastrointestinal mucosa is probably sustained by antigens associated with normal microbial flora and provided the first suggestion that IBD is due to a dysregulated immune response to such antigens, rather than to a particular intestinal pathogen. The finding that disease occurs in SAMP1/Yit mice under pathogen-free but not germ-free conditions is consistent with work in other murine models of mucosal inflammation, where disease, whatever its underlying cause, is only seen when microbial flora are present (7, 8).
In the early 1990s, Elson and his colleagues helped to usher in the modern era of research of animal models of mucosal inflammation with their studies of the diarrhea-prone C3H/HeJBir mouse (9). Effector T cells from these mice mediate a spontaneous pathologic response that results in colitis. These cells react in vivo to elements of the microbial flora and in vitro to certain enteric bacterial antigens (10). Interestingly, only a small subset of the myriad antigens available stimulate humoral responses in these animals, suggesting that some bacterial antigens are more important in disease pathogenesis than others. Indeed, recent work shows that individual organisms in the bacterial microflora differ in their capacity to induce inflammation and that some bacteria actually prevent inflammation, perhaps by crowding out more harmful bacteria. Thus, lactobacillus species prevent colitis in IL-10–deficient mice, which would ordinarily develop colitis under pathogen-free conditions, whereas Bacteroides vulgatus and Helicobacter hepaticus induce inflammation in certain rodent models (11, 12). Duchmann et al. (13, 14) have linked these mouse data to human disease by showing that T cells in both species normally respond to heterologous microflora, but not to organisms found in the host’s own digestive tract. These authors showed that disease-associated T cells isolated from either the inflamed gastric mucosa of the mouse or the active lesions of patients with Crohn’s disease mount proliferative responses to autologous microbial antigens, indicating that, in both cases, inflammatory diseases involve a loss of tolerance to normal microflora.
Common themes from diverse models
Over the past ten years, a cornucopia of models of mucosal inflammation have become available for study, each providing unique insights into the pathogenesis of human IBD (15). These include spontaneous disease models, such as the C3H/HeJBir and SAMP1/Yit mice, as well as a spontaneous colitis model in a primate, the cotton-top tamarin. In other models, disease is induced by exogenous agents such as trinitrobenzene sulfonic acid, oxazolone, dextran sulfate, or peptidoglycan polysaccharide. Finally, mice with specific immune defects can result in colitis, as is seen in mutants carrying deletions in the genes for IL-2, IL-10, or Gαi2 and in another model based on the transfer of specific T-cell subpopulations to SCID and Rag-2–deficient mice.
In most of these models, as in patients with Crohn’s disease, Th1 T cells, are activated by IL-12 and can be found in the inflammatory lesions, where they drive local inflammation by producing IFN-γ and TNF-α (15). This common pathogenesis suggests that Crohn’s disease, too, might arise from a number of different underlying immune abnormalities but that it should be treatable using either agents that block the Th1 response cascade, such as anti–IL-12, or agents that oppose TNF-α, the major downstream cytokine arising from the Th1 response. In fact, anti–IL-12 is now in a clinical trial, and anti–TNF-α (and other TNF antagonists) are already of proven worth in the treatment of Crohn’s disease (16).
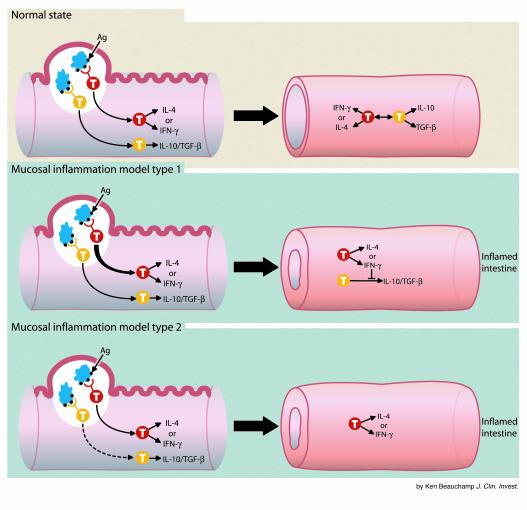
If indeed the various immune defects associated with mucosal inflammation have a final common pathway in an excessive Th1 response, what general factors activate this pathway? Immunologic analyses of the models, as well as knowledge gleaned from the broader field of mucosal immunity, teaches that the Th1 inflammation can be induced either by an excessive Th1 “drive” or an inadequate Th1 “brake,” the latter consisting of a counterregulatory or suppressor response that would normally control excessive mucosal responses (see Figure 1). The importance of such counterregulation can be seen in the development of oral tolerance, wherein exposure to soluble antigens inducing responses in the mucosal immune tissues leads to clonal deletion or anergy of effector T cells or to the induction of suppressor T cells producing TGF-β and/or IL-10 (17).
Figure 1.
Routes to mucosal inflammation. Antigens stimulating the mucosal immune system are presented via dendritic cells (DC; light blue) in the Peyer’s patches. In response, effector T cells (red) produce either IL-4 or IFN-γ and provide defense, whereas regulatory T cells (yellow) produce IL-10 and/or TGF-β and regulate the effector cells. In the normal, uninflamed intestine, the responses by effector cells and regulatory cells are balanced. T-cell development under these conditions (top panel) allows for host defense without inflammation. In the generalized model of mucosal inflammation, type 1 (middle panel), genetic factors lead to an excessive effector cell response that overwhelms or inhibits regulatory responses; inflammation ensues. Conversely, in the generalized model of mucosal inflammation, type 2 (bottom panel), the effector response is intrinsically normal but genetic factors lead to an attenuated regulatory response and, once again, to inflammation. While certain genetic factors must be present for disease to develop, environmental triggers must also be present to bring these genetic factors into play.
Neurath and his colleagues (18) have provided a good example of a colitis model in which an excessive Th1 response drives the disease. Mice that overexpress STAT4, a mediator of IL-12 signaling, develop colitis when immunized with an otherwise innocuous antigen, TNP-KLH. In addition, cells from these transgenic mice, when exposed in vitro to autologous intestinal microflora, can transfer colitis to naive recipients. Thus, in this model, the high rate of colitis reflects a genetically determined increased tendency to respond to mucosal stimulation with a vigorous Th1 response. In the IL-10 knockout mouse and the SCID transfer model, on the other hand, disease results from inadequate counterregulation. Thus, in the absence of IL-10, a molecule that inhibits the Th1 T-cell response primarily by inhibiting IL-12 production, unbalanced Th1 responsiveness clearly underlies the disease (19).
In the SCID transfer model, colitis occurs when mice lacking endogenous lymphoid systems are reconstituted by naive (CD45RBhi) T cells. Upon exposure to intestinal microbes in the recipient animal, these cells apparently give rise to microbial antigen–specific effector Th1 cells (20). Interestingly, colitis does not occur if mature, CD45RBlo T cells are coadministered, indicating that this population includes regulatory cells that can block the Th1 response. CD45RBlo T cells derived from IL-10 knockout mice have no such protective effect, and even wild-type CD45RBlo T cells are ineffective if administered in the presence of a blocking antibody to TGF-β. Thus, peripheral tolerance to normal bacterial epitopes appears to be mediated by IL-10 and TGF-β and to operate through the suppression of Th1 responses (21, 22).
In each of these models, disease occurs because of Th1 T-cell responses, much as is seen in Crohn’s disease. It should be mentioned, however, that several models of colitis have been studied in which Th2 T-cell responses go awry. These include the T-cell receptor α chain knockout mouse, which develops inflammation of the cecum presumably due to a dysregulated response to an organism in this region of the intestine (23), and SJL/J mice administered oxazolone, an agent that induces contact sensitivity (24). These Th2 cell–driven models are histologically more similar to ulcerative colitis than to Crohn’s disease, and they may serve as models of this form of IBD, which is associated with an increased IL-5 response and a normal IFN-γ response (25). However, because no increased IL-4 response is seen in ulcerative colitis, it is premature to call ulcerative colitis a typical Th2 cell–mediated inflammation.
The SAMP1/Yit mouse as a model for Crohn’s disease
In the present study of the SAMP1/Yit mouse, Kosiewicz et al. (1) note that the mucosal inflammation, first described by Matsumoto and his colleagues (3), is centered in the small intestine, is marked by a discontinuous inflammation, and consists of inflammation containing granulomata. These cardinal features of Crohn’s disease are not shared with most previous models, indicating that the SAMP1/Yit mouse is a singular model of the human disease and should yield fundamental insights into its immunopathogenesis. Additionally, since the disease in the SAMP1/Yit mouse is localized to the small intestine, it is likely that the resident organisms in this part of the bowel produce the antigens that cause both the SAMP/Yit phenotype and Crohn’s disease.
The current work also extends our knowledge of the mucosal inflammation in the SAMP1/Yit model in several respects. First, as is seen in Crohn’s disease, T cells present in the mouse lesions produce IFN-γ. Moreover, these cells can be used to transfer disease to normal recipient mice, suggesting that they are indeed the basis of the inflammation in this model. Finally, the authors show, using this adoptive transfer procedure, that disease can largely be prevented by administering anti–TNF-α. The success of this therapy marks yet another significant parallel with human Crohn’s disease and raises the hope that other agents that are found to ameliorate the SAMP1/Yit phenotype will be of use in treating Crohn’s disease.
While the data so far accumulated on the SAMP1/Yit mouse already provide important insights into the nature of this disease of mice, important questions remain to be addressed — as they have been, to varying extents, in other models. Is the Th1 disease that occurs spontaneously in SAMP1/Yit mice the product of an excessive Th1 response, as in the case of STAT4 transgenic mice, or is it caused by an inadequate counterregulation, as in the SCID transfer model? Which organisms in the microflora contribute most to the Th1 response? Are some organisms protective, as has been seen in IL-10–deficient mice? Finally, what are the genetic factors that lead to this remarkable phenotype? SAMP1/Yit mice can be interbred with mice of different genetic backgrounds in order to map and, eventually, isolate the relevant genes involved in SAMP1/Yit colitis. Given the remarkable similarity between this condition and the human disease, the answers to these questions may well apply directly to Crohn’s disease.
References
- 1.Kosiewicz MM, et al. Th1-type responses mediate spontaneous ileitis in a novel murine model of Crohn’s disease. J Clin Invest. 2001;107:695–702. doi: 10.1172/JCI10956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Takeda T, et al. A new murine model of accelerated senescence. Mech Ageing Dev. 1981;17:183–194. doi: 10.1016/0047-6374(81)90084-1. [DOI] [PubMed] [Google Scholar]
- 3.Matsumoto S, et al. Inflammatory bowel disease-like enteritis and caecitis in a senescence accelerated mouse P1/Yit strain. Gut. 1998;43:71–78. doi: 10.1136/gut.43.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Strober W. Animal models of inflammatory bowel disease: an overview. Dig Dis Sci. 1985;30(Suppl.):3S–10S. doi: 10.1007/BF01296964. [DOI] [PubMed] [Google Scholar]
- 5.Kraft S, et al. Histologic and immunohistochemical features of the Auer “colitis” in rabbits. Am J Pathol. 1962;43:913–923. [PMC free article] [PubMed] [Google Scholar]
- 6.Mee AS, et al. Chronic immune colitis in rabbits. Gut. 1979;20:1–5. doi: 10.1136/gut.20.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Contractor NV, et al. Lymphoid hyperplasia, autoimmunity and compromised intestinal intraepithelial lymphocyte development in colitis-free gnotobiotic IL-12 deficient mice. J Immunol. 1998;160:385–394. [PubMed] [Google Scholar]
- 8.Dianda L, et al. T cell receptor-alpha beta-deficient mice fail to develop colitis in the absence of a microbial environment. Am J Pathol. 1997;150:91–97. [PMC free article] [PubMed] [Google Scholar]
- 9.Elson CO, Cong Y, Sundberg J. The C3H/HeJBir mouse model: a high susceptibility phenotype for colitis. Int Rev Immunol. 2000;19:63–75. doi: 10.3109/08830180009048390. [DOI] [PubMed] [Google Scholar]
- 10.Cong Y, et al. CD4+ T cells reactive to enteric bacterial antigens in spontaneous colitic C3H/HeJBir mice: increased T helper cell type 1 response and ability to transfer disease. J Exp Med. 1998;187:855–864. doi: 10.1084/jem.187.6.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Madsen K, et al. Lactobacillus species prevents colitis in interleukin 10 gene-deficient mice. Gastroenterology. 1999;116:1107–1114. doi: 10.1016/s0016-5085(99)70013-2. [DOI] [PubMed] [Google Scholar]
- 12.Sellon RK, et al. Resident enteric bacteria are necessary for development of spontaneous colitis and immune system activation in interleukin-10-deficient mice. Infect Immun. 1998;66:5224–5231. doi: 10.1128/iai.66.11.5224-5231.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duchmann R, et al. Tolerance exists towards resident intestinal flora but is broken in active inflammatory bowel disease (IBD) Clin Exp Immunol. 1995;102:448–455. doi: 10.1111/j.1365-2249.1995.tb03836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duchmann R, et al. Tolerance towards resident intestinal flora in mice is abrogated in experimental colitis and restored by treatment with interleukin-10 or antibodies to interleukin-12. Eur J Immunol. 1996;26:934–938. doi: 10.1002/eji.1830260432. [DOI] [PubMed] [Google Scholar]
- 15.Blumberg RS, Saubermann LJ, Strober W. Animal models of mucosal inflammation and their relation to human inflammatory bowel disease. Curr Opin Immunol. 1999;11:648–656. doi: 10.1016/s0952-7915(99)00032-1. [DOI] [PubMed] [Google Scholar]
- 16.Papadakis KA, Targan SR. Role of cytokines in the pathogenesis of inflammatory bowel disease. Annu Rev Med. 2000;51:289–298. doi: 10.1146/annurev.med.51.1.289. [DOI] [PubMed] [Google Scholar]
- 17.Strober W, Kelsall B, Marth T. Oral tolerance. J Clin Immunol. 1998;18:1–30. doi: 10.1023/a:1023222003039. [DOI] [PubMed] [Google Scholar]
- 18.Wirtz S, et al. Chronic intestinal inflammation in STAT-4 transgenic mice: characterization of disease and adoptive transfer by TNF-plus IFN-gamma-producing CD4+ T cells that respond to bacterial antigens. J Immunol. 1999;162:1884–1888. [PubMed] [Google Scholar]
- 19.Davidson N, et al. IL-12, but not IFN-γ, plays a major role in sustaining the chronic phase of colitis in IL-10-deficient mice. J Immunol. 1998;161:3143–3149. [PubMed] [Google Scholar]
- 20.Powrie F, et al. Phenotypically distinct subsets of CD4+ T cells induce or protect from chronic inflammation in the CB-17 SCID mouse. Int Immunol. 1993;5:1461–1471. doi: 10.1093/intimm/5.11.1461. [DOI] [PubMed] [Google Scholar]
- 21.Powrie F, et al. A critical role for transforming growth factor-beta but not interleukin 4 in the suppression of T helper type 1-mediated colitis by CD45Rblow CD4+ cells. J Exp Med. 1996;183:2669–2674. doi: 10.1084/jem.183.6.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Asseman C, et al. An essential role for interleukin-10 in the function of regulatory T cells that inhibit intestinal inflammation. J Exp Med. 1999;190:995–1004. doi: 10.1084/jem.190.7.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mizoguchi A, Mizoguchi E, Bhan AK. The critical role of interleukin 4 but not interferon gamma in the pathogenesis of colitis in T-cell receptor α mutant mice. Gastroenterology. 1999;116:320–326. doi: 10.1016/s0016-5085(99)70128-9. [DOI] [PubMed] [Google Scholar]
- 24.Boirivant M, et al. Oxazalone colitis: a murine model of T helper cell type 2 colitis treatable with antibodies to interleukin 4. J Exp Med. 1998;188:1929–1939. doi: 10.1084/jem.188.10.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fuss I, et al. Disparate CD4+ lamina propria (LP) lymphokine secretion profiles in inflammatory bowel disease. Crohn’s disease LP cells manifest increased secretion of IFN-gamma, whereas ulcerative colitis LP cells manifest increased secretion of IL-5. J Immunol. 1996;157:1261–1270. [PubMed] [Google Scholar]