Abstract
Transfection of V79 Chinese hamster cells produced clones in which CuZn-superoxide dismutase (CuZn-SOD) activities were 2.2- to 3.5-fold higher than in the parental cells. An overall reduction of antioxidant enzyme activities and both total and oxidized glutathione levels had been found in these clones. Aconitase activities in these cells were determined to indirectly measure the O2− steady-state levels. As expected, in cells overexpressing CuZn-SOD, both total and cytosolic aconitase activities have increased. Because these clones showed reduced oxidized glutathione contents, it is unlikely that they present higher H2O2 steady-state levels as a consequence of the higher SOD levels. This was confirmed by measuring H2O2 steady-state levels in cells by flow cytometric analysis of 2′,7′-dichlorofluorescein diacetate-treated cells. Despite the decreased antioxidant defenses, three of the clones overexpressing CuZn-SOD showed reduced H2O2 steady-state levels. These reduced H2O2 steady-state levels were found even when the cells were treated with the O2− generator 2,3-dimethoxy-1,4-naphthoquinone. These data provide in vivo support for the hypothesis proposed by Liochev and Fridovich [Liochev, S. I. & Fridovich, I. (1994) Free Radical Biol. Med. 16, 29–33] that O2− dismutation prevents the formation of higher H2O2 levels by other reactions.
Keywords: oxidative stress/superoxide anion/glutathione, 2′,7′-dichlorofluorescein diacetate/2,3-dimethoxy-1,4-naphthoquinone
The physiological role of superoxide dismutase (SOD) in aerobic organisms has been discussed since the characterization of its enzymatic activity (1). Many efforts have been made to understand this role and also the mechanisms of the superoxide anion (O2−) toxicity as reviewed by many authors (2–6).
One of the strategies that has been widely used to understand O2− toxicity is the production of SOD overexpressing cell lines, as reviewed elsewhere (7, 8). In some cases, the overexpression of SOD in mammalian systems produced a higher sensitivity to reactive oxygen species (9, 10) also observed in bacteria (11, 12). This has been attributed to an accumulation of the O2− dismutation product, H2O2, caused by SOD overexpression (9–11). In fact, there are two reports that show higher H2O2 steady-state levels in CuZn-SOD-overexpressing cells (13, 14).
However, as proposed by Liochev and Fridovich (3, 15), an excess of SOD should decrease the steady-state O2− level without increasing the endogenous formation of H2O2. They discussed that a more efficient O2− dismutation system would, in fact, prevent the formation of higher stoichiometric H2O2 levels by other reactions. Among them are the formation of 1 mol of H2O2 per mol of O2− in the oxidation of the iron–sulfur clusters of aconitase and other dehydratases—as recently reviewed (4)—and also the formation of even higher H2O2 yields when O2− acts as an initiator in free radical chain oxidations involving biomolecules, such as catecholamines and dihydroflavins. In addition to the several explanations exposed by Liochev and Fridovich (3), we also have hypothesized that as there is no evidence for the increase in the rate of O2− formation in SOD-overexpressing cells, there is also no possibility that these cells present higher H2O2 producing rates. This can be explained by the accepted idea of steady-state condition, according to which there would be no way of increasing H2O2 steady-state concentration if there were no increase in the supplying rate of O2− or decrease in the decay rate of H2O2.
To test whether the SOD overexpression could lead to variations in H2O2 and O2− steady-state levels, we derived cell variants overexpressing CuZn-SOD. Lower O2− steady-state levels were indirectly determined in CuZn-SOD-overexpressing clones by measuring their aconitase activities. H2O2 steady-state levels in these clones were determined by flow cytometric analysis by using the probe 2′,7′-dichlorofluorescein diacetate (DCFH-DA). CuZn-SOD-overexpressing clones presented reduced H2O2 steady-state levels, and, interestingly, these reduced H2O2 levels were observed even under oxidative stress.
MATERIALS AND METHODS
Cell Culture.
M8 is a clone derived from V79 Chinese hamster lung fibroblast. CuZn-SOD overexpressing clones were obtained as described previously (8). The cells were grown in DMEM, pH 7.0, supplemented with 10% (vol/vol) fetal calf serum, 472 units of penicillin/ml, and 94 μg of streptomycin/ml. The cells were cultured in humidified CO2/air (1:19) at 37°C. CuZn-SOD overexpressing cells were cultured in the presence of 500 μg/ml geneticin, the antibiotic used to select these clones, except for a 48-h period prior to the assay.
Reagents.
DCFH-DA was obtained from Molecular Probes and was used as a 5 mM dimethyl sulfoxide solution. Superoxide anion generator 2,3-dimethoxy-1,4-naphthoquinone (DMNQ) was kindly provided by Dr. Enrique Cadenas and was used as a 10 mM ethanolic solution.
Cell Extracts.
Cells were trypsinized, resuspended in DMEM containing 10% (vol/vol) fetal calf serum, and washed once with phosphate-buffered saline solution and then with the extraction buffer (0.25 M sucrose/10 mM Tris/0.1 mM EDTA/2 mM sodium citrate/1 mM succinic acid, pH 7.4). After a 5-min centrifugation at 500 g at 4°C, the pellet was resuspended in extraction buffer containing either 0.2% (wt/vol) Nonidet P-40 or 0.0075% (wt/vol) digitonin for the determination of total or cytosolic aconitase activity, respectively. The extracts were then immediately centrifuged at 14,000 × g for 10 min at 4°C. The supernatants were transferred to fresh tubes and bubbled with N2 to remove oxygen. This procedure was performed on ice and as fast as possible to avoid aconitase inactivation. The supernatants were also used for protein determination (16).
Antioxidant Enzyme Assays.
CuZn-SOD, Mn-SOD, catalase, glutathione peroxidase, glutathione reductase, and glucose-6-phosphate dehydrogenase activities were assayed as described previously (8).
Glutathione Determination.
Both total and oxidized glutathione (GSSG) levels were determined as described previously (8). GSSG content was expressed in terms of GSSG/total glutathione as a weight ratio.
Aconitase Assay.
Aconitase activity was determined in fresh extracts as described by Gardner and White (17). The assay was performed in a 1.0-ml reaction mixture containing 50 mM Tris, 5 mM sodium citrate, 0.6 mM MnCl2, 0.2 mM NADP+, pH 7.4, and 0.5 units of porcine heart isocitrate dehydrogenase. Reaction was started by the addition of the cell extracts. The mixture was incubated for 7 min at 25°C, after which the linear absorbance change at 340 nm at 25°C was measured for 3 min. This initial incubation period is necessary for the accumulation of cis-aconitate (18), especially in assays containing low aconitase activity (17).
Determination of Intracellular H2O2 Steady-State Levels.
Cells (∼1 × 106) were trypsinized and resuspended in ice-cold Hank’s balanced salt solution containing 0.2 mg/ml of soybean trypsin inhibitor. Cell suspensions were then mixed with equal volumes of Hank’s balanced salt solution containing 10 μM DCFH-DA and incubated for 10 min at 37°C. Aliquots of 1.104 cells were then immediately scanned on a FACSTARPLUS (Beckton Dickinson) with excitation and emission settings of 504 and 529 nm, respectively. Histograms were analyzed with software program WinMDI 2.5.
Kinetics of H2O2 Formation After Treatment with 100 μM DMNQ.
Cells (∼5 × 106) were trypsinized and resuspended in ice-cold Hank’s balanced salt solution containing 0.2 mg/ml of soybean trypsin inhibitor. Cell suspensions were preincubated for 5 min at 37°C and then treated with 100 μM DMNQ at 37°C. Aliquots were taken at different times, mixed with equal volumes of Hank’s balanced salt solution containing 10 μM DCFH-DA, and further incubated for 5 min at 37°C. Cellular fluorescence was then immediately determined by flow cytometric analysis as described above.
RESULTS
CuZn-SOD-overexpressing clones were obtained by cell transfection as described previously (8). The four pGSOD clones showed an increase in CuZn-SOD activity when compared with the parental cell line, M8 (Table 1). These clones showed also an increase in CuZn-SOD mRNA levels as described previously (8). No differences were observed in Mn-SOD activity (Table 1). Further biochemical characterization of these clones revealed that statistically significant decreases (P < 0.05) occurred for some antioxidant enzyme activities (Table 2) and also for both total and oxidized glutathione levels (Table 3).
Table 1.
Relative SOD activities in transfected cells
| Cell lines | CuZn-SOD | Mn-SOD |
|---|---|---|
| M8 | 1.00 ± 0.05 (5) | 1.00 ± 0.17 (11) |
| pGSOD1 | 2.30 ± 0.11 (5)* | 0.90 ± 0.03 (11) |
| pGSOD2 | 2.25 ± 0.25 (5)* | 1.08 ± 0.12 (11) |
| pGSOD3 | 2.25 ± 0.07 (5)* | 0.99 ± 0.10 (11) |
| pGSOD4 | 3.55 ± 0.68 (5)* | 1.08 ± 0.05 (11) |
SOD activities are expressed relative to M8 cells. The values correspond to the mean ± SD for the number of determinations indicated in parentheses. M8 cells: SOD contents, CuZn-SOD, 5.6 ± 0.3 units/mg protein; Mn-SOD, 0.565 ± 0.095 units/mg protein (∗, P < 0.05 compared with M8 cells as determined by Student’s t test).
Table 2.
Relative enzymatic antioxidant contents
| Cell lines | Catalase | Glutathione peroxidase | Glutathione reductase | Glucose-6-phosphate dehydrogenase |
|---|---|---|---|---|
| M8 | 1.00 ± 0.04 (8) | 1.00 ± 0.04 (6) | 1.00 ± 0.02 (8) | 1.00 ± 0.01 (7) |
| pGSOD1 | 1.00 ± 0.04 (8) | 0.86 ± 0.02 (6) | 0.90 ± 0.01 (6)* | 0.87 ± 0.01 (6)* |
| pGSOD2 | 1.04 ± 0.04 (8) | 0.77 ± 0.07 (6)* | 0.97 ± 0.04 (6) | 0.89 ± 0.01 (6)* |
| pGSOD3 | 1.11 ± 0.08 (8) | 0.85 ± 0.05 (6)* | 0.92 ± 0.01 (6)* | 0.84 ± 0.02 (6)* |
| pGSOD4 | 0.52 ± 0.04 (8)* | 0.94 ± 0.05 (6) | 0.77 ± 0.01 (6)* | 0.79 ± 0.01 (6)* |
Enzyme activities are expressed relative to M8 cells. The values correspond to the mean ± SD for the number of determinations indicated in parentheses. Antioxidant contents of M8 cells: catalase, 27 ± 1 units/mg protein; glutathione peroxidase, 2.42 ± 0.09 units/g protein; glutathione reductase, 99 ± 2 units/g protein; and glucose-6-phosphate dehydrogenase, 145 ± 1 units/g protein (∗, P < 0.05 compared with M8 cells as determined by Student’s t test).
Table 3.
Relative glutathione contents
| Cell lines | Total glutathione | GSSG/total glutathione |
|---|---|---|
| M8 | 1.00 ± 0.17 (15) | 1.00 ± 0.02 (14) |
| pGSOD1 | 0.74 ± 0.07 (11)* | 0.80 ± 0.05 (9)* |
| pGSOD2 | 0.59 ± 0.17 (11)* | 0.77 ± 0.03 (9)* |
| pGSOD3 | 0.78 ± 0.22 (14) | 0.76 ± 0.07 (9)* |
| pGSOD4 | 0.32 ± 0.12 (10)* | 0.73 ± 0.13 (10)* |
Total glutathione contents and GSSG/total glutathione ratios are expressed relative to M8 cells. The values correspond to the mean ± SD for the number of determinations indicated in parentheses. Glutathione contents of M8 cells: total glutathione, 6.7 ± 1.1 mg/g protein; GSSG/total glutathione, 0.0114 ± 0.0002 g of GSSG/g of total glutathione (∗, P < 0.05 compared with M8 cells as determined by Student’s t test).
It is well established that aconitase is inactivated in vivo by O2− (19, 20) and that its activity can be used to indirectly estimate intracellular O2− steady-state concentrations (17, 21). To determine if the increased CuZn-SOD activity really leads to decreases in O2− steady-state concentrations in these clones, we have measured their aconitase activities. As expected, CuZn-SOD-overexpressing clones presented higher aconitase activities when compared with the parental cell line (Table 4), demonstrating that, indeed, these clones present lower O2− steady-state levels. The higher increases in cytosolic aconitase activities may result from the cytosolic localization of the overexpressed enzyme. We were not able, however, to estimate variations in O2− steady-state concentrations in the different cell lines. This impossibility is a consequence of the differences in glutathione levels in the clones (Table 3). It is known that reduced glutathione is required for the process of aconitase reactivation (22) and the method for calculating O2− steady-state concentrations assumes that there are no changes in glutathione levels (17, 19, 21).
Table 4.
Relative aconitase activities
| Cell lines | Total aconitase | Cytosolic aconitase |
|---|---|---|
| M8 | 1.00 ± 0.01 (6) | 1.00 ± 0.07 (7) |
| pGSOD1 | 1.13 ± 0.03 (6)* | 1.11 ± 0.09 (7)* |
| pGSOD2 | 1.11 ± 0.02 (7)* | 1.25 ± 0.06 (7)* |
| pGSOD3 | 1.19 ± 0.07 (7)* | 1.20 ± 0.05 (7)* |
| pGSOD4 | 1.16 ± 0.04 (6)* | 1.30 ± 0.03 (5)* |
Aconitase activities are expressed relative to M8 cells. The values correspond to the mean ± SD for the number of determinations indicated in parentheses. Aconitase activities of M8 cells: total aconitase, 10.23 ± 0.11 units/g protein; cytosolic aconitase, 3.79 ± 0.27 units/g protein (∗, P < 0.05 compared with M8 cells as determined by Student’s t test).
Because the clones we obtained showed reduced GSSG contents (Table 3), it is unlikely that they present higher H2O2 steady-state levels as a consequence of the higher CuZn-SOD levels. This was confirmed by measuring H2O2 steady-state levels in cells by flow cytometric analysis of DCFH-DA-treated cells. This probe is used for determination of intracellular peroxide levels (13, 14, 23). Despite the decreased antioxidant defenses, clones pGSOD1, -2, and -3 showed statistically significant reductions in H2O2 steady-state levels (Table 5).
Table 5.
Relative intracellular H2O2 steady-state levels
| Cell lines | Relative DCFH-DA fluorescence |
|---|---|
| M8 | 1 |
| pGSOD1 | 0.83 ± 0.08 (4)* |
| pGSOD2 | 0.83 ± 0.11 (4)* |
| pGSOD3 | 0.81 ± 0.10 (3)* |
| pGSOD4 | 0.86 ± 0.19 (4) |
Intracellular H2O2 steady-state levels are expressed relative to M8 cells. The values correspond to the mean ± SD for the number of determinations indicated in parentheses (∗, P < 0.05 compared with M8 cells as determined by Student’s t test).
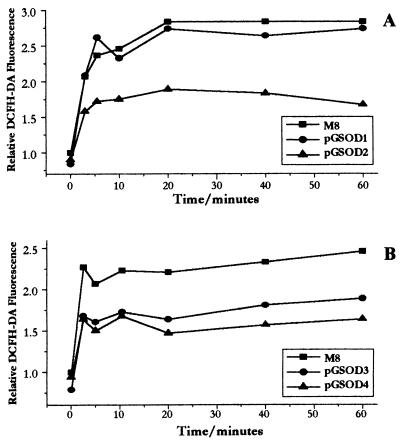
We also determined the H2O2 levels in these cells under oxidative stressing conditions by treating them with DMNQ. DMNQ is a well known O2− intracellular generator that is unable to undergo any type of alkylation reactions, hence, contributing to cytotoxicity only by redox cycling (24–26). We noticed then that reduced H2O2 levels were found in CuZn-SOD-overexpressing clones even when the clones were treated with DMNQ, under conditions where the H2O2 formation is immediately increased (Fig. 1). The increase in O2− concentrations were confirmed by an 80% reduction in M8 total aconitase activity after a 20-min treatment with 100 μM DMNQ (data not shown). To obtain statistically significant data, we have determined these H2O2 levels after a fixed time of incubation with DMNQ (Table 6). This confirms that these clones present statistically significant decreases in H2O2 levels when compared with DMNQ-treated parental cell line.
Figure 1.
Kinetics of H2O2 formation after treatment with 100 μM DMNQ. Cell suspensions were treated with 100 μM DMNQ. Aliquots were taken at different times and further incubated with the probe DCFH-DA for 5 min. Cellular fluorescence was then immediately determined by flow cytometric analysis. (A and B) Results of representative kinetics assays.
Table 6.
Intracellular H2O2 levels in DMNQ-treated cells
| Cell lines | Relative DCFH-DA fluorescence
|
|
|---|---|---|
| Control | 100 μM DMNQ | |
| M8 | 1.00 | 1.93 ± 0.18 |
| pGSOD1 | 0.86 ± 0.12 | 1.63 ± 0.19 |
| pGSOD2 | 0.72 ± 0.11 | 1.19 ± 0.17* |
| pGSOD3 | 0.70 ± 0.08 | 1.20 ± 0.09* |
| pGSOD4 | 0.77 ± 0.14 | 1.33 ± 0.21* |
Cell suspensions were treated for 20 min with 100 μM DMNQ at 37°C by the addition of an ethanolic concentrated solution. Control cells were treated with the same volume of ethanol. Cells were further incubated with the probe DCFH-DA for 5 min at 37°C, and the cellular fluorescence was then determined by flow cytometric analysis. The values correspond to the mean ± SD for three independent experiments (∗, P < 0.05 compared with DMNQ-treated M8 cells as determined by Student’s t test).
DISCUSSION
The overexpression of CuZn-SOD has been used as investigation models of degenerative processes like familial amyotrophic lateral sclerosis (27–29), Down syndrome (10, 30, 31), and aging (32, 33). It is well known that in mammalian cells this overexpression has caused a large variety of responses in terms of sensitivity to oxidative stress (7, 9, 10, 14, 34, 35). Many types of adaptations have been observed involving the expression of antioxidant enzymes and other proteins that are not directly involved with the cellular homeostasis of reactive oxygen species. There is, however, a lack of data on how SOD overexpression affects the cellular content of its substrate and product, respectively O2− and H2O2. As discussed in the Introduction, there are at least two opposite hypotheses regarding the H2O2 level in SOD overexpressing cells. In the present report, we attempted to provide new elements for this discussion.
All four clones investigated in the present work showed higher aconitase activities when compared with the parental cell line (Table 4). The clone that has the highest CuZn-SOD activity, pGSOD4, presents also, as expected, the highest increase in the cytosolic aconitase activity. These results demonstrate that these clones undergo a decrease in the O2− steady-state levels. It is interesting to notice that, because reduced glutathione is necessary in the process of aconitase reactivation (22), the observed increases in aconitase activities could be even higher if the CuZn-SOD-overexpressing clones presented the same glutathione amounts of the parental cell line.
The characterization of the four CuZn-SOD-overexpressing clones showed a global decrease in other antioxidant species (Tables 2 and 3), suggesting an up-regulation of a group of genes by O2− as previously discussed (8). It is very important to notice that these decreases affect almost all elements of the cellular H2O2 consuming system. As a result of this observation, it is improbable that these clones have been adapted to conditions of higher H2O2 steady-state levels. This was confirmed by accessing the H2O2 steady-state levels by flow cytometric analysis, using the probe DCFH-DA (Table 5). Due to its ester groups, this probe is an uncharged molecule that can permeate living cell membranes (36, 37). It is then hydrolyzed by intracellular esterases to a charged molecule, 2′,7′-dichlorofluorescin, that is trapped inside the cell. The oxidation of the 2′,7′-dichlorofluorescin is induced by intracellular peroxides (13, 38, 39), in a reaction catalyzed by peroxidases (40). This oxidation leads to the formation of the high fluorescent molecule 2′,7′-dichlorofluorescein (41). Because of these properties, the DCFH-DA has been widely used as an intracellular H2O2 probe (13, 36, 37, 39, 42–45). This lower H2O2 steady-state in pGSOD clones seems to be even more relevant if we take into account that these clones present a decrease in the cellular H2O2 consuming system (Tables 2 and 3). These decreases in H2O2 levels were also determined under oxidative stressing conditions, when cells were pretreated with the intracellular O2−-generator DMNQ (Fig. 1 and Table 6). It is also important to notice that pGSOD4 clone, which has the lowest levels of glutathione and catalase, also shows lower H2O2 levels after DMNQ treatment (Fig. 1B and Table 6). When the cells are treated with DMNQ, the intracellular O2− generation rate is increased (24–26). At least theoretically, however, this rate is the same in the clones, and, as we proposed earlier, there is no reason for the increase in the production of H2O2, unless if there is an increase in the rate of production of the SOD substrate, O2−. It is important to notice that these reductions in H2O2, reported for pGSOD clones, are not caused by decreases in cellular DCFH-DA esterases and/or peroxidases, as we have determined that the extracts of all clones present the same ability to oxidize DCFH-DA in vitro under conditions of excess of H2O2 (data not shown). This decrease in H2O2 levels has not been detected previously in SOD overexpressing lines. There are two cases in which an increase in H2O2 levels was detected (13, 14). The reasons for these differences are not obvious. One possibility is to consider that these lines somehow responded to the SOD overexpression by increasing the H2O2 generation by other sources as, for instance, by activating H2O2-generating oxidases. This could be a reasonable explanation because it has been recently demonstrated that H2O2 is necessary for many physiological cellular control processes, including signal transduction and gene expression (13, 42, 45–50).
If we consider together all these variations in the cellular H2O2-consuming system (Tables 2 and 3), the lower levels of GSSG (Table 3), and the lower H2O2 levels detected in the pGSOD clones (Tables 5 and 6; Fig. 1), we may reason that the CuZn-SOD-overexpression does not necessarily lead to higher H2O2 levels. These data represent in vivo evidence for the hypothesis proposed by Liochev and Fridovich (3) that an excess of SOD would act in a way of preventing the formation of higher stoichiometric H2O2 levels by other reactions. We have also observed an increased reducing environment in pGSOD clones as denoted by the lowering in GSSG and H2O2 levels. This also provides support for another hypothesis of Liochev and Fridovich (3, 4) that a decrease in O2− steady-state concentration would lead to a decrease in the rate of consumption of cellular reductants.
Acknowledgments
This work was supported by grants from Fundaçao de Amparo à Pesquisa do Estado de Sao Paulo, Conselho Nacional de Pesquisa e Desenvolvimento (Brazil), Comissao de Aperfeiçoamento de Pessoal de Nival Superior, and Financiadora de Estudos e Projetos (Brazilian agencies of support to science). H.D.T. holds a fellowship from Fundaçao de Amparo à Pesquisa do Estado de Sao Paulo.
ABBREVIATIONS
- SOD
superoxide dismutase
- DCFH-DA
2′,7′-dichlorofluorescein diacetate
- DMNQ
2,3-dimethoxy-1,4-naphthoquinone
- GSSG
oxidized glutathione
References
- 1.McCord J M, Fridovich I. J Biol Chem. 1969;244:6049–6055. [PubMed] [Google Scholar]
- 2.Fridovich I. Annu Rev Biochem. 1995;64:97–112. doi: 10.1146/annurev.bi.64.070195.000525. [DOI] [PubMed] [Google Scholar]
- 3.Liochev S I, Fridovich I. Free Radical Biol Med. 1994;16:29–33. doi: 10.1016/0891-5849(94)90239-9. [DOI] [PubMed] [Google Scholar]
- 4.Liochev S I. Free Radical Res. 1996;25:369–384. doi: 10.3109/10715769609149059. [DOI] [PubMed] [Google Scholar]
- 5.Meneghini R, Benfato M S, Bertoncini C R A, Carvalho H, Gurgueira S, Robalinho R L, Teixeira H D, Wendel C M A, Nascimento A L T O. Cancer J. 1995;8:109–113. [Google Scholar]
- 6.Cadenas E. Annu Rev Biochem. 1989;58:79–110. doi: 10.1146/annurev.bi.58.070189.000455. [DOI] [PubMed] [Google Scholar]
- 7.Kelner M J, Bagnell R, Montoya M, Estes L, Uglik S F, Cerutti P. Free Radical Biol Med. 1995;18:497–506. doi: 10.1016/0891-5849(94)00167-i. [DOI] [PubMed] [Google Scholar]
- 8.Teixeira H D, Meneghini R. Biochem J. 1996;315:821–825. doi: 10.1042/bj3150821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Amstad P, Peskin A, Shah G, Mirault M, Moret R, Zbinden I, Cerutti P. Biochemistry. 1991;30:9305–9313. doi: 10.1021/bi00102a024. [DOI] [PubMed] [Google Scholar]
- 10.Elroy-Stein O, Bernstein Y, Groner Y. EMBO J. 1986;5:615–622. doi: 10.1002/j.1460-2075.1986.tb04255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scott M D, Meshnick S R, Eaton J W. J Biol Chem. 1989;264:2498–2501. [PubMed] [Google Scholar]
- 12.Scott M D, Meshnick S R, Eaton J W. J Biol Chem. 1987;262:3640–3645. [PubMed] [Google Scholar]
- 13.Schmidt K N, Amstad P, Cerutti P, Baeuerle P A. Chem Biol. 1995;2:13–22. doi: 10.1016/1074-5521(95)90076-4. [DOI] [PubMed] [Google Scholar]
- 14.De Haan J B, Cristiano F, Iannello R, Bladier C, Kelner M J, Kola I. Hum Mol Genet. 1996;5:283–292. doi: 10.1093/hmg/5.2.283. [DOI] [PubMed] [Google Scholar]
- 15.Liochev S I, Fridovich I. J Biol Chem. 1991;266:8747–8750. [PubMed] [Google Scholar]
- 16.Hartree E F. Anal Biochem. 1972;48:422–427. doi: 10.1016/0003-2697(72)90094-2. [DOI] [PubMed] [Google Scholar]
- 17.Gardner P R, White C W. In: The Oxygen Paradox. Davies K J A, editor. Padua: Univ. of Padua Press; 1994. pp. 33–50. [Google Scholar]
- 18.Krebs H A, Holzach O. Biochem J. 1952;52:527–528. doi: 10.1042/bj0520527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gardner P R, Raineri I, Epstein L B, White C W. J Biol Chem. 1995;270:13399–13405. doi: 10.1074/jbc.270.22.13399. [DOI] [PubMed] [Google Scholar]
- 20.Gardner P R, Fridovich I. J Biol Chem. 1991;266:19328–19333. [PubMed] [Google Scholar]
- 21.Gardner P R, Fridovich I. J Biol Chem. 1992;267:8757–8763. [PubMed] [Google Scholar]
- 22.Gardner P R, Fridovich I. Arch Biochem Biophys. 1993;301:98–102. doi: 10.1006/abbi.1993.1120. [DOI] [PubMed] [Google Scholar]
- 23.Boissy R E, Trinkle L S, Nordlund J J. Cytometry. 1989;10:779–787. doi: 10.1002/cyto.990100616. [DOI] [PubMed] [Google Scholar]
- 24.Brunmark A, Cadenas E. Free Radical Biol Med. 1989;7:435–477. doi: 10.1016/0891-5849(89)90126-3. [DOI] [PubMed] [Google Scholar]
- 25.Shi M, Gozal E, Choy H A, Forman H J. Free Radical Biol Med. 1993;15:57–67. doi: 10.1016/0891-5849(93)90125-e. [DOI] [PubMed] [Google Scholar]
- 26.Henry T R, Wallace K B. Arch Toxicol. 1996;70:482–489. doi: 10.1007/s002040050302. [DOI] [PubMed] [Google Scholar]
- 27.Dal Canto M C, Gurney M E. Brain Res. 1995;676:25–40. doi: 10.1016/0006-8993(95)00063-v. [DOI] [PubMed] [Google Scholar]
- 28.Rabizadeh S, Gralla E B, Borchelt D R, Gwinn R, Valentine J S, Sisodia S, Wong P, Lee M, Hahn H, Bredesen D E. Proc Natl Acad Sci USA. 1995;92:3024–3028. doi: 10.1073/pnas.92.7.3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peled-Kamar M, Lotem J, Wirguin I, Weiner L, Hermalin A, Groner Y. Proc Natl Acad Sci USA. 1997;94:3883–3887. doi: 10.1073/pnas.94.8.3883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Elroy-Stein O, Groner Y. Cell. 1988;52:259–267. doi: 10.1016/0092-8674(88)90515-6. [DOI] [PubMed] [Google Scholar]
- 31.Minc-Golomb D, Knobler H, Groner Y. EMBO J. 1991;10:2119–2124. doi: 10.1002/j.1460-2075.1991.tb07745.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Orr W C, Sohal R S. Science. 1994;263:1128–1130. doi: 10.1126/science.8108730. [DOI] [PubMed] [Google Scholar]
- 33.Reveillaud I, Niedzwiecki A, Bensch K G, Fleming J E. Mol Cell Biol. 1991;11:632–640. doi: 10.1128/mcb.11.2.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Amstad P, Moret R, Cerutti P. J Biol Chem. 1994;269:1606–1609. [PubMed] [Google Scholar]
- 35.Kelner M J, Bagnell R. J Biol Chem. 1990;265:10872–10875. [PubMed] [Google Scholar]
- 36.Bass D A, Parce J W, Dechatelet L R, Szjeda P, Seeds M S, Thomas M. J Immunol. 1983;130:1910–1917. [PubMed] [Google Scholar]
- 37.Szejda P, Parce J W, Seeds M S, Bass D A. J Immunol. 1984;133:3303–3307. [PubMed] [Google Scholar]
- 38.Yuan L, Inoue S, Saito Y, Nakajima O. Exp Cell Res. 1993;209:375–381. doi: 10.1006/excr.1993.1323. [DOI] [PubMed] [Google Scholar]
- 39.Ubezio P, Civoli F. Free Radical Biol Med. 1994;16:509–516. doi: 10.1016/0891-5849(94)90129-5. [DOI] [PubMed] [Google Scholar]
- 40.Cathcart R, Schwiers E, Ames B N. Anal Biochem. 1983;134:111–116. doi: 10.1016/0003-2697(83)90270-1. [DOI] [PubMed] [Google Scholar]
- 41.Keston A S, Brandt R. Anal Biochem. 1965;11:1–5. doi: 10.1016/0003-2697(65)90034-5. [DOI] [PubMed] [Google Scholar]
- 42.Sundaresan M, Yu Z X, Ferrans V J, Irani K, Finkel T. Science. 1995;270:296–299. doi: 10.1126/science.270.5234.296. [DOI] [PubMed] [Google Scholar]
- 43.Kane D J, Sarafian T A, Anton R, Hahn H, Gralla E B, Valentine J S, Örd T, Bredesen D E. Science. 1993;262:1274–1277. doi: 10.1126/science.8235659. [DOI] [PubMed] [Google Scholar]
- 44.Mirochnitchenko O, Inouye M. J Immunol. 1996;156:1578–1586. [PubMed] [Google Scholar]
- 45.Bae Y S, Kang S W, Seo M S, Baines I C, Tekle E, Chock P B, Rhee S G. J Biol Chem. 1997;272:217–221. [PubMed] [Google Scholar]
- 46.Martins E A L, Robalinho R L, Meneghini R. Arch Biochem Biophys. 1995;316:128–134. doi: 10.1006/abbi.1995.1019. [DOI] [PubMed] [Google Scholar]
- 47.Burdon R H. Biochem Soc Trans. 1996;24:1028–1032. doi: 10.1042/bst0241028. [DOI] [PubMed] [Google Scholar]
- 48.Pantopoulos K, Hentze M W. EMBO J. 1995;14:2917–2924. doi: 10.1002/j.1460-2075.1995.tb07291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Suzuki Y J, Forman H J, Sevanian A. Free Radical Biol Med. 1997;22:269–285. doi: 10.1016/s0891-5849(96)00275-4. [DOI] [PubMed] [Google Scholar]
- 50.Monteiro H P, Stern A. Free Radical Biol Med. 1996;21:323–333. doi: 10.1016/0891-5849(96)00051-2. [DOI] [PubMed] [Google Scholar]