Abstract
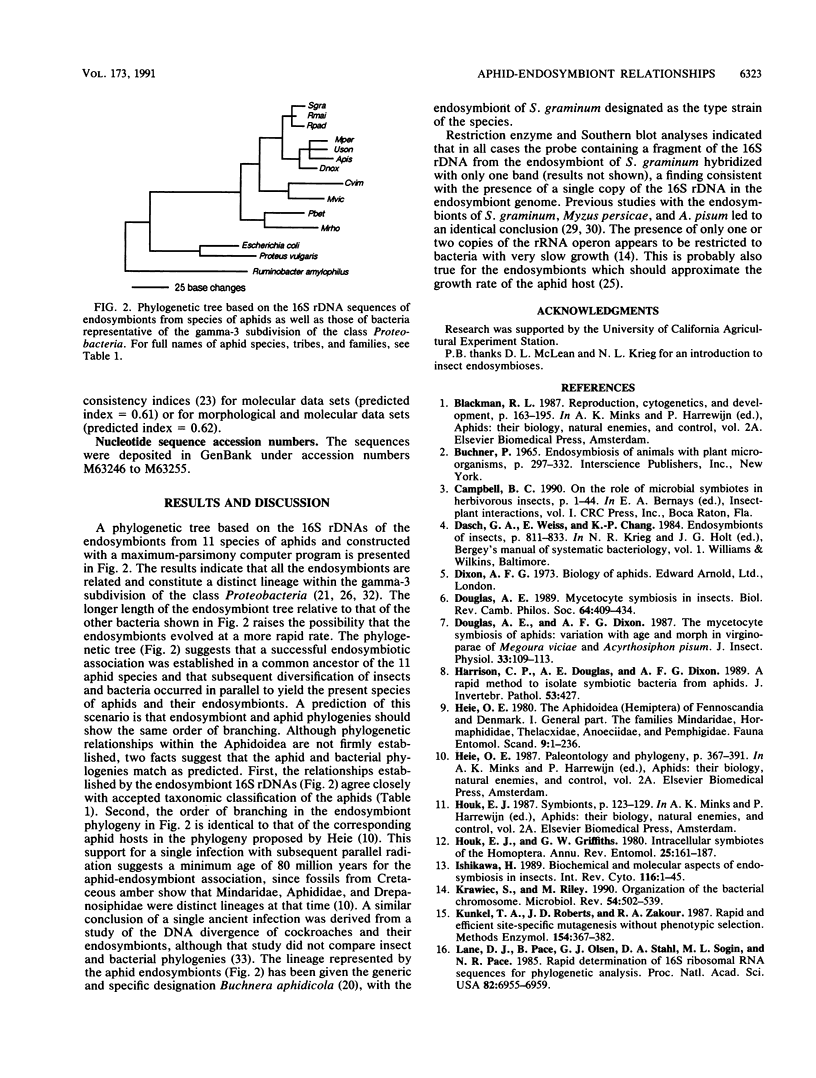
Aphids (superfamily Aphidoidea) contain eubacterial endosymbionts localized within specialized cells (mycetocytes). The endosymbionts are essential for the survival of the aphid hosts. Sequence analyses of the 16S rRNAs from endosymbionts of 11 aphid species from seven tribes and four families have indicated that the endosymbionts are monophyletic. Furthermore, phylogenetic relationships within the symbiont clade parallel the relationships of the corresponding aphid hosts. Our findings suggest that this endocytobiotic association was established in a common ancestor of the four aphid families with subsequent diversification into the present species of aphids and their endosymbionts.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Douglas A. E. Mycetocyte symbiosis in insects. Biol Rev Camb Philos Soc. 1989 Nov;64(4):409–434. doi: 10.1111/j.1469-185x.1989.tb00682.x. [DOI] [PubMed] [Google Scholar]
- Ishikawa H. Biochemical and molecular aspects of endosymbiosis in insects. Int Rev Cytol. 1989;116:1–45. doi: 10.1016/s0074-7696(08)60637-3. [DOI] [PubMed] [Google Scholar]
- Krawiec S., Riley M. Organization of the bacterial chromosome. Microbiol Rev. 1990 Dec;54(4):502–539. doi: 10.1128/mr.54.4.502-539.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel T. A., Roberts J. D., Zakour R. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- Lane D. J., Pace B., Olsen G. J., Stahl D. A., Sogin M. L., Pace N. R. Rapid determination of 16S ribosomal RNA sequences for phylogenetic analyses. Proc Natl Acad Sci U S A. 1985 Oct;82(20):6955–6959. doi: 10.1073/pnas.82.20.6955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unterman B. M., Baumann P., McLean D. L. Pea aphid symbiont relationships established by analysis of 16S rRNAs. J Bacteriol. 1989 Jun;171(6):2970–2974. doi: 10.1128/jb.171.6.2970-2974.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeks D. P., Beerman N., Griffith O. M. A small-scale five-hour procedure for isolating multiple samples of CsCl-purified DNA: application to isolations from mammalian, insect, higher plant, algal, yeast, and bacterial sources. Anal Biochem. 1986 Feb 1;152(2):376–385. doi: 10.1016/0003-2697(86)90423-9. [DOI] [PubMed] [Google Scholar]
- Woese C. R. Bacterial evolution. Microbiol Rev. 1987 Jun;51(2):221–271. doi: 10.1128/mr.51.2.221-271.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]



