Abstract
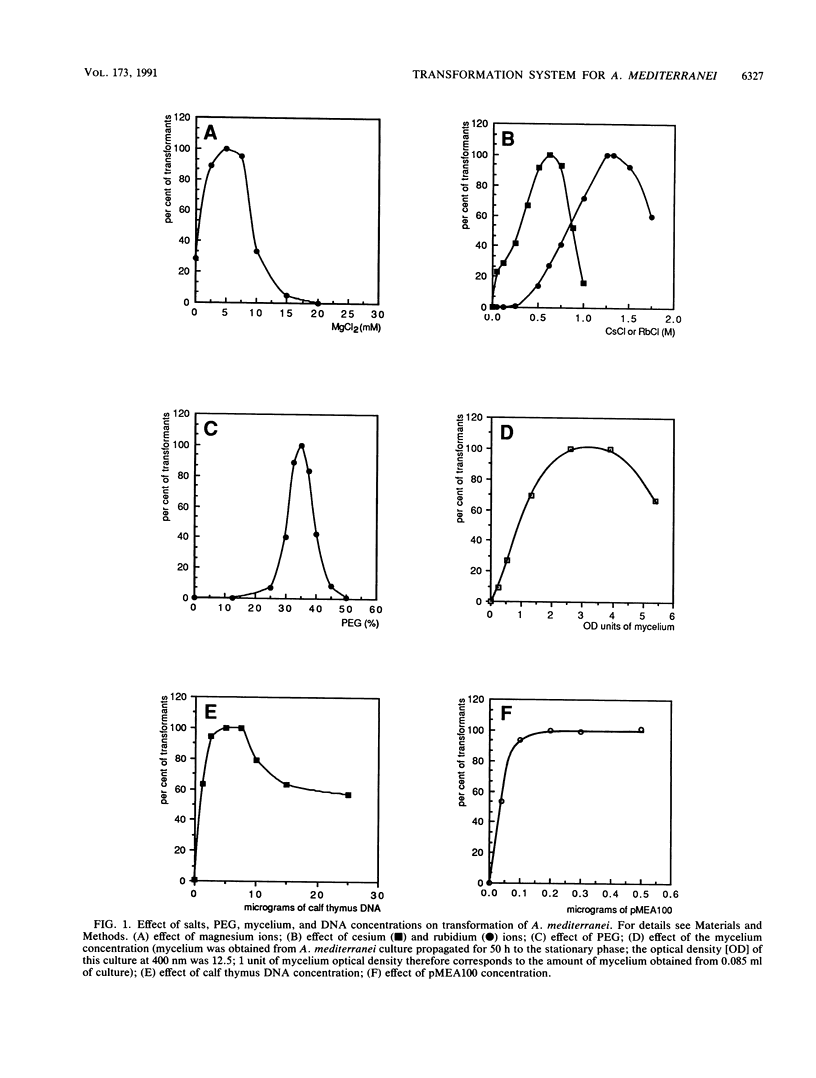
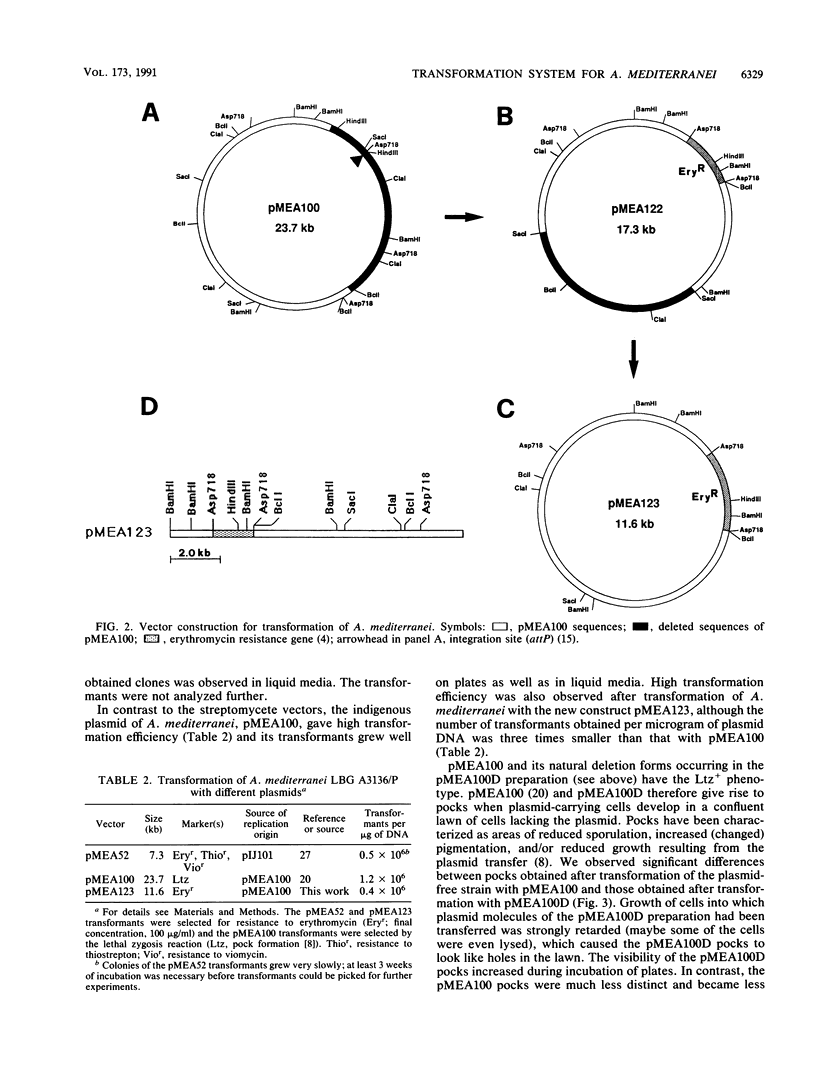
A new procedure for transformation of Amycolatopsis (Nocardia) mediterranei LBG A3136 was developed. The method makes use of polyethylene glycol and alkaline cations and enables direct transformation of the A. mediterranei mycelium with high efficiency: more than 10(6) transformants per microgram of DNA were obtained. Transformation of A. mediterranei is stimulated by the ionophore antibiotic valinomycin and abolished by arsenate and p-chloromercuribenzenesulfonate. pMEA123, a vector based on the indigenous plasmid pMEA100 and containing the erythromycin resistance gene, was constructed.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baltz R. H. Genetic recombination in Streptomyces fradiae by protoplast fusion and cell regeneration. J Gen Microbiol. 1978 Jul;107(1):93–102. doi: 10.1099/00221287-107-1-93. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya P., Epstein W., Silver S. Valinomycin-induced uptake of potassium in membrane vesicles from Escherichia coli. Proc Natl Acad Sci U S A. 1971 Jul;68(7):1488–1492. doi: 10.1073/pnas.68.7.1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boccard F., Smokvina T., Pernodet J. L., Friedmann A., Guérineau M. The integrated conjugative plasmid pSAM2 of Streptomyces ambofaciens is related to temperate bacteriophages. EMBO J. 1989 Mar;8(3):973–980. doi: 10.1002/j.1460-2075.1989.tb03460.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hintermann G., Crameri R., Vögtli M., Hütter R. Streptomycin-sensitivity in Streptomyces glaucescens is due to deletions comprising the structural gene coding for a specific phosphotransferase. Mol Gen Genet. 1984;196(3):513–520. doi: 10.1007/BF00436201. [DOI] [PubMed] [Google Scholar]
- Ito H., Fukuda Y., Murata K., Kimura A. Transformation of intact yeast cells treated with alkali cations. J Bacteriol. 1983 Jan;153(1):163–168. doi: 10.1128/jb.153.1.163-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieser T., Hopwood D. A., Wright H. M., Thompson C. J. pIJ101, a multi-copy broad host-range Streptomyces plasmid: functional analysis and development of DNA cloning vectors. Mol Gen Genet. 1982;185(2):223–228. doi: 10.1007/BF00330791. [DOI] [PubMed] [Google Scholar]
- Klebe R. J., Harriss J. V., Sharp Z. D., Douglas M. G. A general method for polyethylene-glycol-induced genetic transformation of bacteria and yeast. Gene. 1983 Nov;25(2-3):333–341. doi: 10.1016/0378-1119(83)90238-x. [DOI] [PubMed] [Google Scholar]
- Klein W. L., Boyer P. D. Energization of active transport by Escherichia coli. J Biol Chem. 1972 Nov 25;247(22):7257–7265. [PubMed] [Google Scholar]
- Lombardi F. J., Reeves J. P., Kaback H. R. Mechanisms of active transport in isolated bacterial membrane vesicles. 8. Valinomycin-induced rubidium transport. J Biol Chem. 1973 May 25;248(10):3551–3565. [PubMed] [Google Scholar]
- Madon J., Moretti P., Hütter R. Site-specific integration and excision of pMEA100 in Nocardia mediterranei. Mol Gen Genet. 1987 Sep;209(2):257–264. doi: 10.1007/BF00329651. [DOI] [PubMed] [Google Scholar]
- Malpartida F., Zalacaín M., Jiménez A., Davies J. Molecular cloning and expression in streptomyces lividans of a hygromycin B phosphotransferase gene from Streptomyces hygroscopicus. Biochem Biophys Res Commun. 1983 Nov 30;117(1):6–12. doi: 10.1016/0006-291x(83)91533-4. [DOI] [PubMed] [Google Scholar]
- Matsushima P., McHenney M. A., Baltz R. H. Efficient transformation of Amycolatopsis orientalis (Nocardia orientalis) protoplasts by Streptomyces plasmids. J Bacteriol. 1987 May;169(5):2298–2300. doi: 10.1128/jb.169.5.2298-2300.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moretti P., Hintermann G., Hütter R. Isolation and characterization of an extrachromosomal element from Nocardia mediterranei. Plasmid. 1985 Sep;14(2):126–133. doi: 10.1016/0147-619x(85)90072-1. [DOI] [PubMed] [Google Scholar]
- Ovchinnikov Y. A. Physico-chemical basis of ion transport through biological membranes: ionophores and ion channels. Eur J Biochem. 1979 Mar;94(2):321–336. doi: 10.1111/j.1432-1033.1979.tb12898.x. [DOI] [PubMed] [Google Scholar]
- PRIDHAM T. G., ANDERSON P., FOLEY C., LINDENFELSER L. A., HESSELTINE C. W., BENEDICT R. G. A selection of media for maintenance and taxonomic study of Streptomyces. Antibiot Annu. 1956:947–953. [PubMed] [Google Scholar]
- Sabelnikov A. G., Domaradsky I. V. Effect of metabolic inhibitors on entry of exogenous deoxyribonucleic acid into Ca2+-treated Escherichia coli cells. J Bacteriol. 1981 May;146(2):435–443. doi: 10.1128/jb.146.2.435-443.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto H., Maurer K. H., Hutchinson C. R. Transformation of Streptomyces erythraeus. J Antibiot (Tokyo) 1986 Sep;39(9):1304–1313. doi: 10.7164/antibiotics.39.1304. [DOI] [PubMed] [Google Scholar]
- Zhu B., Madoń J., Häusler A., Hütter R. Amplification on the Amycolatopsis (Nocardia) mediterranei plasmid pMEA100: sequence similarities to actinomycete att sites. Plasmid. 1990 Sep;24(2):132–142. doi: 10.1016/0147-619x(90)90015-5. [DOI] [PubMed] [Google Scholar]




