Abstract
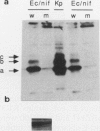
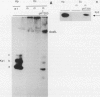
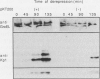
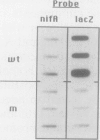
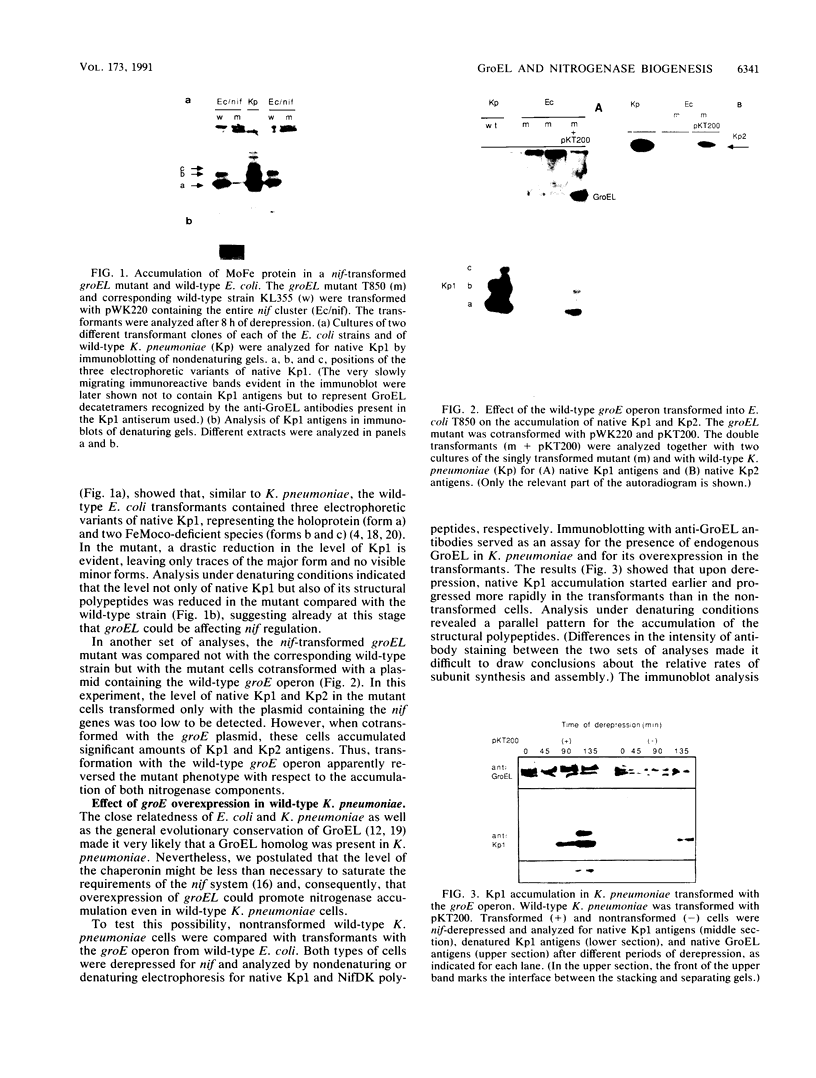
Several approaches were used to study the role of GroEL, the prototype chaperonin, in the nitrogen fixation (nif) system. An Escherichia coli groEL mutant transformed with the Klebsiella pneumoniae nif gene cluster accumulated very low to nondetectable levels of nitrogenase components compared with the isogenic wild-type strain or the mutant cotransformed with the wild-type groE operon. In K. pneumoniae, overexpression of the E. coli groE operon markedly accelerated the rate of appearance of the MoFe protein and its constituent polypeptides after the start of derepression. The groEL mutation in E. coli decreased NifA-dependent beta-galactosidase expression from the nifH promoter but did not affect the constitutive expression of nifA from the tet promoter of ntr-controlled expression from the nifLA promoter. The possibility that GroEL is required for the correct folding of NifA was supported by coimmunoprecipitation of NifA with anti-GroEL antibodies. Kinetic analyses of nitrogenase assembly in 35S pulse-chased K. pneumoniae pointed to the existence of high-molecular-weight intermediates in MoFe protein assembly and demonstrated the transient binding of newly synthesized NifH and NifDK to GroEL. Overall, these results indicate that GroEL fulfills both regulatory and structural functions in the nif system.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnold W., Rump A., Klipp W., Priefer U. B., Pühler A. Nucleotide sequence of a 24,206-base-pair DNA fragment carrying the entire nitrogen fixation gene cluster of Klebsiella pneumoniae. J Mol Biol. 1988 Oct 5;203(3):715–738. doi: 10.1016/0022-2836(88)90205-7. [DOI] [PubMed] [Google Scholar]
- Austin S., Henderson N., Dixon R. Characterisation of the Klebsiella pneumoniae nitrogen-fixation regulatory proteins NIFA and NIFL in vitro. Eur J Biochem. 1990 Jan 26;187(2):353–360. doi: 10.1111/j.1432-1033.1990.tb15312.x. [DOI] [PubMed] [Google Scholar]
- Berman J., Gershoni J. M., Zamir A. Expression of nitrogen fixation genes in foreign hosts. Assembly of nitrogenase Fe protein in Escherichia coli and in yeast. J Biol Chem. 1985 May 10;260(9):5240–5243. [PubMed] [Google Scholar]
- Bitoun R., Berman J., Zilberstein A., Holland D., Cohen J. B., Givol D., Zamir A. Promoter mutations that allow nifA-independent expression of the nitrogen fixation nifHDKY operon. Proc Natl Acad Sci U S A. 1983 Oct;80(19):5812–5816. doi: 10.1073/pnas.80.19.5812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom M., Skelly S., VanBogelen R., Neidhardt F., Brot N., Weissbach H. In vitro effect of the Escherichia coli heat shock regulatory protein on expression of heat shock genes. J Bacteriol. 1986 May;166(2):380–384. doi: 10.1128/jb.166.2.380-384.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bochkareva E. S., Lissin N. M., Girshovich A. S. Transient association of newly synthesized unfolded proteins with the heat-shock GroEL protein. Nature. 1988 Nov 17;336(6196):254–257. doi: 10.1038/336254a0. [DOI] [PubMed] [Google Scholar]
- Brooks S. J., Collins J. J., Brill W. J. Repression of nitrogen fixation in Klebsiella pneumoniae at high temperature. J Bacteriol. 1984 Feb;157(2):460–464. doi: 10.1128/jb.157.2.460-464.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan-Wollaston V., Cannon M. C., Beynon J. L., Cannon F. C. Role of the nifA gene product in the regulation of nif expression in Klebsiella pneumoniae. Nature. 1981 Dec 24;294(5843):776–778. doi: 10.1038/294776a0. [DOI] [PubMed] [Google Scholar]
- Ellis R. J. The molecular chaperone concept. Semin Cell Biol. 1990 Feb;1(1):1–9. [PubMed] [Google Scholar]
- Fayet O., Louarn J. M., Georgopoulos C. Suppression of the Escherichia coli dnaA46 mutation by amplification of the groES and groEL genes. Mol Gen Genet. 1986 Mar;202(3):435–445. doi: 10.1007/BF00333274. [DOI] [PubMed] [Google Scholar]
- Fayet O., Ziegelhoffer T., Georgopoulos C. The groES and groEL heat shock gene products of Escherichia coli are essential for bacterial growth at all temperatures. J Bacteriol. 1989 Mar;171(3):1379–1385. doi: 10.1128/jb.171.3.1379-1385.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgopoulos C., Ang D. The Escherichia coli groE chaperonins. Semin Cell Biol. 1990 Feb;1(1):19–25. [PubMed] [Google Scholar]
- Goloubinoff P., Gatenby A. A., Lorimer G. H. GroE heat-shock proteins promote assembly of foreign prokaryotic ribulose bisphosphate carboxylase oligomers in Escherichia coli. Nature. 1989 Jan 5;337(6202):44–47. doi: 10.1038/337044a0. [DOI] [PubMed] [Google Scholar]
- Gosink M. M., Franklin N. M., Roberts G. P. The product of the Klebsiella pneumoniae nifX gene is a negative regulator of the nitrogen fixation (nif) regulon. J Bacteriol. 1990 Mar;172(3):1441–1447. doi: 10.1128/jb.172.3.1441-1447.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govezensky D., Zamir A. Structure-function relationships in the alpha subunit of Klebsiella pneumoniae nitrogenase MoFe protein from analysis of nifD mutants. J Bacteriol. 1989 Oct;171(10):5729–5735. doi: 10.1128/jb.171.10.5729-5735.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmingsen S. M., Woolford C., van der Vies S. M., Tilly K., Dennis D. T., Georgopoulos C. P., Hendrix R. W., Ellis R. J. Homologous plant and bacterial proteins chaperone oligomeric protein assembly. Nature. 1988 May 26;333(6171):330–334. doi: 10.1038/333330a0. [DOI] [PubMed] [Google Scholar]
- Holland D., Zilberstein A., Govezensky D., Salomon D., Zamir A. Nitrogenase MoFe protein subunits from Klebsiella pneumoniae expressed in foreign hosts. Characteristics and interactions. J Biol Chem. 1987 Jun 25;262(18):8814–8820. [PubMed] [Google Scholar]
- Hoover T. R., Santero E., Porter S., Kustu S. The integration host factor stimulates interaction of RNA polymerase with NIFA, the transcriptional activator for nitrogen fixation operons. Cell. 1990 Oct 5;63(1):11–22. doi: 10.1016/0092-8674(90)90284-l. [DOI] [PubMed] [Google Scholar]
- Howard K. S., McLean P. A., Hansen F. B., Lemley P. V., Koblan K. S., Orme-Johnson W. H. Klebsiella pneumoniae nifM gene product is required for stabilization and activation of nitrogenase iron protein in Escherichia coli. J Biol Chem. 1986 Jan 15;261(2):772–778. [PubMed] [Google Scholar]
- Kalnins A., Otto K., Rüther U., Müller-Hill B. Sequence of the lacZ gene of Escherichia coli. EMBO J. 1983;2(4):593–597. doi: 10.1002/j.1460-2075.1983.tb01468.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumamoto C. A. Escherichia coli SecB protein associates with exported protein precursors in vivo. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5320–5324. doi: 10.1073/pnas.86.14.5320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusukawa N., Yura T., Ueguchi C., Akiyama Y., Ito K. Effects of mutations in heat-shock genes groES and groEL on protein export in Escherichia coli. EMBO J. 1989 Nov;8(11):3517–3521. doi: 10.1002/j.1460-2075.1989.tb08517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Morett E., Buck M. NifA-dependent in vivo protection demonstrates that the upstream activator sequence of nif promoters is a protein binding site. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9401–9405. doi: 10.1073/pnas.85.24.9401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash H. A., Robertson C. A. Purification and properties of the Escherichia coli protein factor required for lambda integrative recombination. J Biol Chem. 1981 Sep 10;256(17):9246–9253. [PubMed] [Google Scholar]
- Ostermann J., Horwich A. L., Neupert W., Hartl F. U. Protein folding in mitochondria requires complex formation with hsp60 and ATP hydrolysis. Nature. 1989 Sep 14;341(6238):125–130. doi: 10.1038/341125a0. [DOI] [PubMed] [Google Scholar]
- Riedel G. E., Brown S. E., Ausubel F. M. Nitrogen fixation by Klebsiella pneumoniae is inhibited by certain multicopy hybrid nif plasmids. J Bacteriol. 1983 Jan;153(1):45–56. doi: 10.1128/jb.153.1.45-56.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts G. P., MacNeil T., MacNeil D., Brill W. J. Regulation and characterization of protein products coded by the nif (nitrogen fixation) genes of Klebsiella pneumoniae. J Bacteriol. 1978 Oct;136(1):267–279. doi: 10.1128/jb.136.1.267-279.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santero E., Hoover T., Keener J., Kustu S. In vitro activity of the nitrogen fixation regulatory protein NIFA. Proc Natl Acad Sci U S A. 1989 Oct;86(19):7346–7350. doi: 10.1073/pnas.86.19.7346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano T., Kakefuda T. Involvement of a bacterial factor in morphogenesis of bacteriophage capsid. Nat New Biol. 1972 Sep 13;239(89):34–37. doi: 10.1038/newbio239034a0. [DOI] [PubMed] [Google Scholar]
- Tuli R., Merrick M. J. Over-production and characterization of the nifA gene product of Klebsiella pneumoniae--the transcriptional activator of nif gene expression. J Gen Microbiol. 1988 Feb;134(2):425–432. doi: 10.1099/00221287-134-2-425. [DOI] [PubMed] [Google Scholar]