Abstract
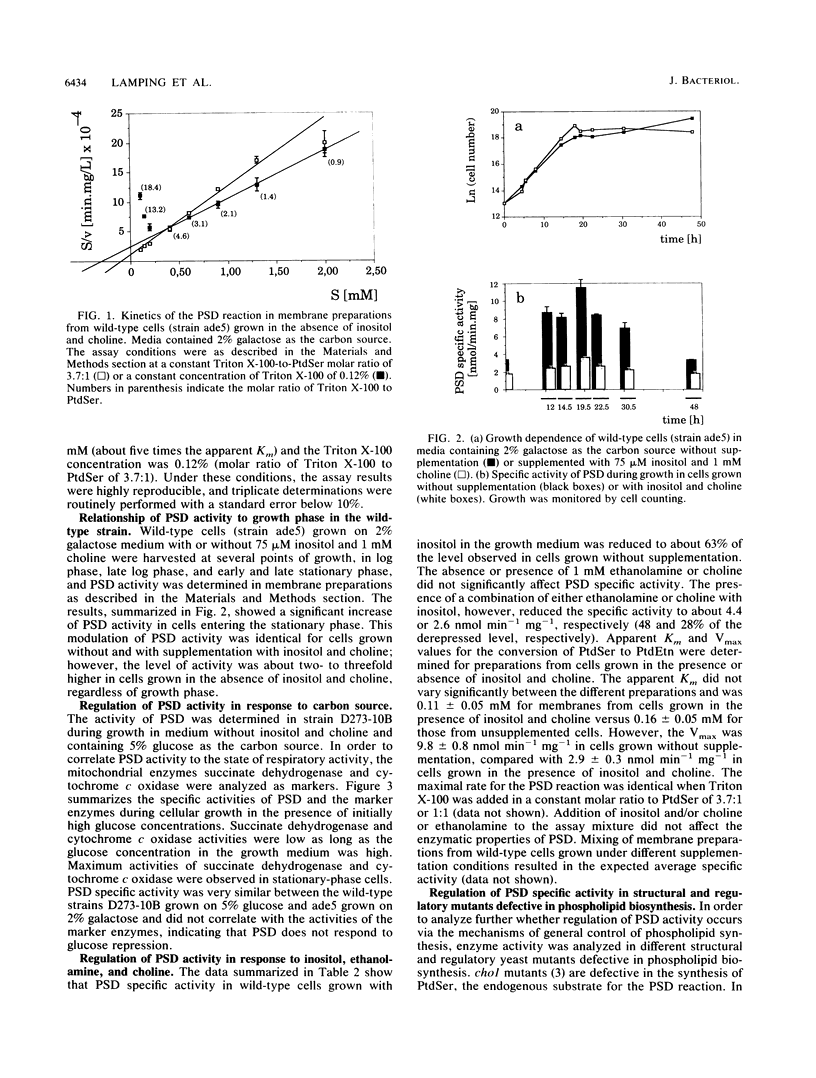
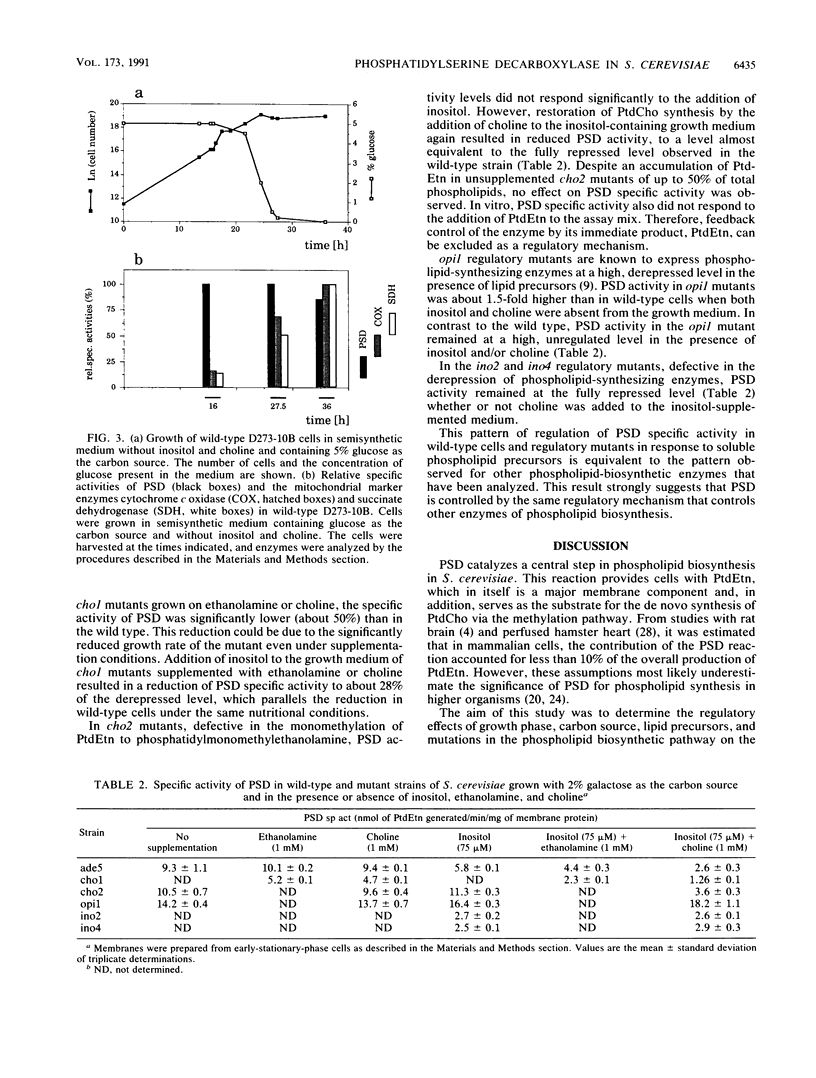
Regulation of the activity of the mitochondrial enzyme phosphatidylserine decarboxylase (PSD) was measured in vitro by using membrane preparations from wild-type and mutant strains of Saccharomyces cerevisiae. PSD specific activity was not affected by carbon source, and on all carbon sources, the highest specific activity was observed in cells entering the stationary phase of growth. However, PSD activity was found to be regulated in response to soluble precursors of phospholipid biosynthesis. PSD specific activity was reduced to about 63% of the level observed in unsupplemented wild-type cells when the cells were grown in the presence of 75 microM inositol. The presence of 1 mM choline alone had no repressing effect, but the presence of 1 mM choline and 75 microM inositol together led to further repression to a level of about 28% of the derepressed activity. Regulatory mutations known to affect regulation or expression of genes encoding phospholipid-synthesizing enzymes also affected PSD specific activity. opi1 mutants, which are constitutive for a number of phospholipid-biosynthetic enzymes, were found to have high, constitutive levels of PSD. Likewise, in ino2 or ino4 regulatory mutants, PSD activity was found to be at the fully repressed level regardless of growth condition. Regulation of PSD activity was also affected in several structural-gene mutants under conditions of impaired phosphatidylcholine biosynthesis. Together, these data strongly suggest that PSD expression is controlled by the mechanism of general control of phospholipid biosynthesis that regulates many enzymes of phospholipid biosynthesis.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackrell B. A., Kearney E. B., Singer T. P. Mammalian succinate dehydrogenase. Methods Enzymol. 1978;53:466–483. doi: 10.1016/s0076-6879(78)53050-4. [DOI] [PubMed] [Google Scholar]
- Bae-Lee M. S., Carman G. M. Phosphatidylserine synthesis in Saccharomyces cerevisiae. Purification and characterization of membrane-associated phosphatidylserine synthase. J Biol Chem. 1984 Sep 10;259(17):10857–10862. [PubMed] [Google Scholar]
- Bailis A. M., Poole M. A., Carman G. M., Henry S. A. The membrane-associated enzyme phosphatidylserine synthase is regulated at the level of mRNA abundance. Mol Cell Biol. 1987 Jan;7(1):167–176. doi: 10.1128/mcb.7.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler M., Morell P. The role of phosphatidylserine decarboxylase in brain phospholipid metabolism. J Neurochem. 1983 Nov;41(5):1445–1454. doi: 10.1111/j.1471-4159.1983.tb00844.x. [DOI] [PubMed] [Google Scholar]
- Carman G. M., Henry S. A. Phospholipid biosynthesis in yeast. Annu Rev Biochem. 1989;58:635–669. doi: 10.1146/annurev.bi.58.070189.003223. [DOI] [PubMed] [Google Scholar]
- Carson M. A., Emala M., Hogsten P., Waechter C. J. Coordinate regulation of phosphatidylserine decarboxylase activity and phospholipid N-methylation in yeast. J Biol Chem. 1984 May 25;259(10):6267–6273. [PubMed] [Google Scholar]
- Culbertson M. R., Henry S. A. Inositol-requiring mutants of Saccharomyces cerevisiae. Genetics. 1975 May;80(1):23–40. doi: 10.1093/genetics/80.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daum G., Heidorn E., Paltauf F. Intracellular transfer of phospholipids in the yeast, Saccharomyces cerevisiae. Biochim Biophys Acta. 1986 Aug 14;878(1):93–101. doi: 10.1016/0005-2760(86)90347-4. [DOI] [PubMed] [Google Scholar]
- Greenberg M. L., Reiner B., Henry S. A. Regulatory mutations of inositol biosynthesis in yeast: isolation of inositol-excreting mutants. Genetics. 1982 Jan;100(1):19–33. doi: 10.1093/genetics/100.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry S. A., Klig L. S., Loewy B. S. The genetic regulation and coordination of biosynthetic pathways in yeast: amino acid and phospholipid synthesis. Annu Rev Genet. 1984;18:207–231. doi: 10.1146/annurev.ge.18.120184.001231. [DOI] [PubMed] [Google Scholar]
- Hirsch J. P., Henry S. A. Expression of the Saccharomyces cerevisiae inositol-1-phosphate synthase (INO1) gene is regulated by factors that affect phospholipid synthesis. Mol Cell Biol. 1986 Oct;6(10):3320–3328. doi: 10.1128/mcb.6.10.3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homann M. J., Henry S. A., Carman G. M. Regulation of CDP-diacylglycerol synthase activity in Saccharomyces cerevisiae. J Bacteriol. 1985 Sep;163(3):1265–1266. doi: 10.1128/jb.163.3.1265-1266.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homann M. J., Poole M. A., Gaynor P. M., Ho C. T., Carman G. M. Effect of growth phase on phospholipid biosynthesis in Saccharomyces cerevisiae. J Bacteriol. 1987 Feb;169(2):533–539. doi: 10.1128/jb.169.2.533-539.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klig L. S., Homann M. J., Carman G. M., Henry S. A. Coordinate regulation of phospholipid biosynthesis in Saccharomyces cerevisiae: pleiotropically constitutive opi1 mutant. J Bacteriol. 1985 Jun;162(3):1135–1141. doi: 10.1128/jb.162.3.1135-1141.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuchler K., Daum G., Paltauf F. Subcellular and submitochondrial localization of phospholipid-synthesizing enzymes in Saccharomyces cerevisiae. J Bacteriol. 1986 Mar;165(3):901–910. doi: 10.1128/jb.165.3.901-910.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Loewy B. S., Henry S. A. The INO2 and INO4 loci of Saccharomyces cerevisiae are pleiotropic regulatory genes. Mol Cell Biol. 1984 Nov;4(11):2479–2485. doi: 10.1128/mcb.4.11.2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason T. L., Poyton R. O., Wharton D. C., Schatz G. Cytochrome c oxidase from bakers' yeast. I. Isolation and properties. J Biol Chem. 1973 Feb 25;248(4):1346–1354. [PubMed] [Google Scholar]
- McGraw P., Henry S. A. Mutations in the Saccharomyces cerevisiae opi3 gene: effects on phospholipid methylation, growth and cross-pathway regulation of inositol synthesis. Genetics. 1989 Jun;122(2):317–330. doi: 10.1093/genetics/122.2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller M. A., Kent C. Characterization of the pathways for phosphatidylethanolamine biosynthesis in Chinese hamster ovary mutant and parental cell lines. J Biol Chem. 1986 Jul 25;261(21):9753–9761. [PubMed] [Google Scholar]
- Poole M. A., Homann M. J., Bae-Lee M. S., Carman G. M. Regulation of phosphatidylserine synthase from Saccharomyces cerevisiae by phospholipid precursors. J Bacteriol. 1986 Nov;168(2):668–672. doi: 10.1128/jb.168.2.668-672.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperka-Gottlieb C., Fasch E. V., Kuchler K., Bailis A. M., Henry S. A., Paltauf F., Kohlwein S. D. The hydrophilic and acidic N-terminus of the integral membrane enzyme phosphatidylserine synthase is required for efficient membrane insertion. Yeast. 1990 Jul-Aug;6(4):331–343. doi: 10.1002/yea.320060406. [DOI] [PubMed] [Google Scholar]
- Summers E. F., Letts V. A., McGraw P., Henry S. A. Saccharomyces cerevisiae cho2 mutants are deficient in phospholipid methylation and cross-pathway regulation of inositol synthesis. Genetics. 1988 Dec;120(4):909–922. doi: 10.1093/genetics/120.4.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voelker D. R. Phosphatidylserine functions as the major precursor of phosphatidylethanolamine in cultured BHK-21 cells. Proc Natl Acad Sci U S A. 1984 May;81(9):2669–2673. doi: 10.1073/pnas.81.9.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waechter C. J., Lester R. L. Differential regulation of the N-methyl transferases responsible for phosphatidylcholine synthesis in Saccharomyces cerevisiae. Arch Biochem Biophys. 1973 Sep;158(1):401–410. doi: 10.1016/0003-9861(73)90637-1. [DOI] [PubMed] [Google Scholar]
- Warner T. G., Dennis E. A. Action of the highly purified, membrane-bound enzyme phosphatidylserine decarboxylase Escherichia coli toward phosphatidylserine in mixed micelles and erythrocyte ghosts in the presence of surfactant. J Biol Chem. 1975 Oct 25;250(20):8004–8009. [PubMed] [Google Scholar]
- Yamashita S., Oshima A. Regulation of phosphatidylethanolamine methyltransferase level by myo-inositol in Saccaromyces cerevisiae. Eur J Biochem. 1980 Mar;104(2):611–616. doi: 10.1111/j.1432-1033.1980.tb04465.x. [DOI] [PubMed] [Google Scholar]
- Zelinski T. A., Choy P. C. Phosphatidylethanolamine biosynthesis in isolated hamster heart. Can J Biochem. 1982 Aug;60(8):817–823. doi: 10.1139/o82-102. [DOI] [PubMed] [Google Scholar]
- Zinser E., Sperka-Gottlieb C. D., Fasch E. V., Kohlwein S. D., Paltauf F., Daum G. Phospholipid synthesis and lipid composition of subcellular membranes in the unicellular eukaryote Saccharomyces cerevisiae. J Bacteriol. 1991 Mar;173(6):2026–2034. doi: 10.1128/jb.173.6.2026-2034.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]


