Abstract
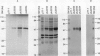
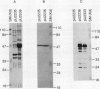
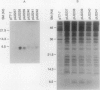
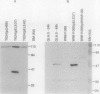
Three proteins, including the beta-keto acyl synthase and the acyl carrier protein, involved in the synthesis of the polyketide antibiotic tetracenomycin C by Streptomyces glaucescens GLA.0 were produced in Escherichia coli by using the T7 RNA polymerase-dependent pT7-7 expression vector. Changing the N-terminal codon usage of two of the genes greatly increased the level of protein produced without affecting mRNA levels, suggesting improvements in translational efficiency. Western immunoblot analysis of cytoplasmic and membrane fractions of S. glaucescens with antibodies raised to synthetic oligopeptides corresponding to the two presumed components of the beta-keto acyl synthase indicated that both proteins were membrane bound; one appears to be proteolytically cleaved before or during association with the membrane. The beta-keto acyl synthase could be detected in stationary-phase cultures but not in rapidly growing cultures, correlating with the time of appearance of tetracenomycin C in the medium.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson S. G., Kurland C. G. Codon preferences in free-living microorganisms. Microbiol Rev. 1990 Jun;54(2):198–210. doi: 10.1128/mr.54.2.198-210.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibb M. J., Biró S., Motamedi H., Collins J. F., Hutchinson C. R. Analysis of the nucleotide sequence of the Streptomyces glaucescens tcmI genes provides key information about the enzymology of polyketide antibiotic biosynthesis. EMBO J. 1989 Sep;8(9):2727–2736. doi: 10.1002/j.1460-2075.1989.tb08414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birch A. J. Biosynthesis of polyketides and related compounds. Science. 1967 Apr 14;156(3772):202–206. doi: 10.1126/science.156.3772.202. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brinkmann U., Mattes R. E., Buckel P. High-level expression of recombinant genes in Escherichia coli is dependent on the availability of the dnaY gene product. Gene. 1989 Dec 21;85(1):109–114. doi: 10.1016/0378-1119(89)90470-8. [DOI] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Caffrey P., Green B., Packman L. C., Rawlings B. J., Staunton J., Leadlay P. F. An acyl-carrier-protein-thioesterase domain from the 6-deoxyerythronolide B synthase of Saccharopolyspora erythraea. High-level production, purification and characterisation in Escherichia coli. Eur J Biochem. 1991 Feb 14;195(3):823–830. doi: 10.1111/j.1432-1033.1991.tb15771.x. [DOI] [PubMed] [Google Scholar]
- Clayton T. M., Bibb M. J. Streptomyces promoter-probe plasmids that utilise the xylE gene of Pseudomonas putida. Nucleic Acids Res. 1990 Feb 25;18(4):1077–1077. doi: 10.1093/nar/18.4.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortes J., Haydock S. F., Roberts G. A., Bevitt D. J., Leadlay P. F. An unusually large multifunctional polypeptide in the erythromycin-producing polyketide synthase of Saccharopolyspora erythraea. Nature. 1990 Nov 8;348(6297):176–178. doi: 10.1038/348176a0. [DOI] [PubMed] [Google Scholar]
- Donadio S., Staver M. J., McAlpine J. B., Swanson S. J., Katz L. Modular organization of genes required for complex polyketide biosynthesis. Science. 1991 May 3;252(5006):675–679. doi: 10.1126/science.2024119. [DOI] [PubMed] [Google Scholar]
- Hopwood D. A., Sherman D. H. Molecular genetics of polyketides and its comparison to fatty acid biosynthesis. Annu Rev Genet. 1990;24:37–66. doi: 10.1146/annurev.ge.24.120190.000345. [DOI] [PubMed] [Google Scholar]
- Hsiung H. M., MacKellar W. C. Expression of bovine growth hormone derivatives in Escherichia coli and the use of the derivatives to produce natural sequence growth hormone by cathepsin C cleavage. Methods Enzymol. 1987;153:390–401. doi: 10.1016/0076-6879(87)53067-1. [DOI] [PubMed] [Google Scholar]
- Ikemura T. Correlation between the abundance of Escherichia coli transfer RNAs and the occurrence of the respective codons in its protein genes: a proposal for a synonymous codon choice that is optimal for the E. coli translational system. J Mol Biol. 1981 Sep 25;151(3):389–409. doi: 10.1016/0022-2836(81)90003-6. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laskey R. A. The use of intensifying screens or organic scintillators for visualizing radioactive molecules resolved by gel electrophoresis. Methods Enzymol. 1980;65(1):363–371. doi: 10.1016/s0076-6879(80)65047-2. [DOI] [PubMed] [Google Scholar]
- Liljenström H., von Heijne G. Translation rate modification by preferential codon usage: intragenic position effects. J Theor Biol. 1987 Jan 7;124(1):43–55. doi: 10.1016/s0022-5193(87)80251-5. [DOI] [PubMed] [Google Scholar]
- Looman A. C., Bodlaender J., Comstock L. J., Eaton D., Jhurani P., de Boer H. A., van Knippenberg P. H. Influence of the codon following the AUG initiation codon on the expression of a modified lacZ gene in Escherichia coli. EMBO J. 1987 Aug;6(8):2489–2492. doi: 10.1002/j.1460-2075.1987.tb02530.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makoff A. J., Oxer M. D., Romanos M. A., Fairweather N. F., Ballantine S. Expression of tetanus toxin fragment C in E. coli: high level expression by removing rare codons. Nucleic Acids Res. 1989 Dec 25;17(24):10191–10202. doi: 10.1093/nar/17.24.10191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motamedi H., Wendt-Pienkowski E., Hutchinson C. R. Isolation of tetracenomycin C-nonproducing Streptomyces glaucescens mutants. J Bacteriol. 1986 Aug;167(2):575–580. doi: 10.1128/jb.167.2.575-580.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn M. J., Gander J. E., Parisi E., Carson J. Mechanism of assembly of the outer membrane of Salmonella typhimurium. Isolation and characterization of cytoplasmic and outer membrane. J Biol Chem. 1972 Jun 25;247(12):3962–3972. [PubMed] [Google Scholar]
- Petersen G. B., Stockwell P. A., Hill D. F. Messenger RNA recognition in Escherichia coli: a possible second site of interaction with 16S ribosomal RNA. EMBO J. 1988 Dec 1;7(12):3957–3962. doi: 10.1002/j.1460-2075.1988.tb03282.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revill W. P., Leadlay P. F. Cloning, characterization, and high-level expression in Escherichia coli of the Saccharopolyspora erythraea gene encoding an acyl carrier protein potentially involved in fatty acid biosynthesis. J Bacteriol. 1991 Jul;173(14):4379–4385. doi: 10.1128/jb.173.14.4379-4385.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynes J. P., Calmels T., Drocourt D., Tiraby G. Cloning, expression in Escherichia coli and nucleotide sequence of a tetracycline-resistance gene from Streptomyces rimosus. J Gen Microbiol. 1988 Mar;134(3):585–598. doi: 10.1099/00221287-134-3-585. [DOI] [PubMed] [Google Scholar]
- Robinson M., Lilley R., Little S., Emtage J. S., Yarranton G., Stephens P., Millican A., Eaton M., Humphreys G. Codon usage can affect efficiency of translation of genes in Escherichia coli. Nucleic Acids Res. 1984 Sep 11;12(17):6663–6671. doi: 10.1093/nar/12.17.6663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp P. M., Li W. H. Codon usage in regulatory genes in Escherichia coli does not reflect selection for 'rare' codons. Nucleic Acids Res. 1986 Oct 10;14(19):7737–7749. doi: 10.1093/nar/14.19.7737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman D. H., Malpartida F., Bibb M. J., Kieser H. M., Bibb M. J., Hopwood D. A. Structure and deduced function of the granaticin-producing polyketide synthase gene cluster of Streptomyces violaceoruber Tü22. EMBO J. 1989 Sep;8(9):2717–2725. doi: 10.1002/j.1460-2075.1989.tb08413.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaink H. P., Weinman J., Djordjevic M. A., Wijffelman C. A., Okker R. J., Lugtenberg B. J. Genetic analysis and cellular localization of the Rhizobium host specificity-determining NodE protein. EMBO J. 1989 Oct;8(10):2811–2818. doi: 10.1002/j.1460-2075.1989.tb08427.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprengart M. L., Fatscher H. P., Fuchs E. The initiation of translation in E. coli: apparent base pairing between the 16srRNA and downstream sequences of the mRNA. Nucleic Acids Res. 1990 Apr 11;18(7):1719–1723. doi: 10.1093/nar/18.7.1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swan D. G., Cortes J., Hale R. S., Leadlay P. F. Cloning, characterization, and heterologous expression of the Saccharopolyspora erythraea (Streptomyces erythraeus) gene encoding an EF-hand calcium-binding protein. J Bacteriol. 1989 Oct;171(10):5614–5619. doi: 10.1128/jb.171.10.5614-5619.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabor S., Richardson C. C. A bacteriophage T7 RNA polymerase/promoter system for controlled exclusive expression of specific genes. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1074–1078. doi: 10.1073/pnas.82.4.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward J. M., Janssen G. R., Kieser T., Bibb M. J., Buttner M. J., Bibb M. J. Construction and characterisation of a series of multi-copy promoter-probe plasmid vectors for Streptomyces using the aminoglycoside phosphotransferase gene from Tn5 as indicator. Mol Gen Genet. 1986 Jun;203(3):468–478. doi: 10.1007/BF00422072. [DOI] [PubMed] [Google Scholar]
- Williams D. P., Regier D., Akiyoshi D., Genbauffe F., Murphy J. R. Design, synthesis and expression of a human interleukin-2 gene incorporating the codon usage bias found in highly expressed Escherichia coli genes. Nucleic Acids Res. 1988 Nov 25;16(22):10453–10467. doi: 10.1093/nar/16.22.10453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue S., Motamedi H., Wendt-Pienkowski E., Hutchinson C. R. Anthracycline metabolites of tetracenomycin C-nonproducing Streptomyces glaucescens mutants. J Bacteriol. 1986 Aug;167(2):581–586. doi: 10.1128/jb.167.2.581-586.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]