Abstract
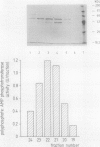
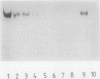
Polyphosphate:AMP phosphotransferase, an enzyme which catalyzes the phosphorylation of AMP to ADP at the expense of polyphosphate, was purified more than 1,500-fold from Acinetobacter strain 210A by streptomycin sulfate precipitation and by Mono-Q, Phenyl Superose, and Superose column chromatography. Streptomycin sulfate precipitation appeared to be an effective step in the purification procedure. During the following chromatographic steps, there was a 29-fold increase in specific activity but the yield was low (0.3%). Kinetic studies showed apparent Km values of 0.26 mM for AMP and 0.8 microM for polyphosphate with an average chain length of 35 phosphate groups. The highest activities were found with polyphosphate molecules of 18 to 44 phosphate residues. The polyphosphate chain was degraded completely to ADP. The mechanism of degradation is processive. No activity was obtained with ortho-, pyro-, tri-, and tetraphosphate. The enzyme was inhibited by pyro-, tri-, and tetraphosphate. The inhibition by tri- and tetraphosphate was mixed with polyphosphate as a substrate. The inhibition constants for the dissociation of the enzyme-inhibitor complex and for the enzyme-inhibitor-substrate complex were 0.9 and 6.5 mM, respectively, for triphosphate and 0.7 and 1.5 mM, respectively, for tetraphosphate.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Clark J. E., Wood H. G. Preparation of standards and determination of sizes of long-chain polyphosphates by gel electrophoresis. Anal Biochem. 1987 Mar;161(2):280–290. doi: 10.1016/0003-2697(87)90452-0. [DOI] [PubMed] [Google Scholar]
- Cornish-Bowden A. A simple graphical method for determining the inhibition constants of mixed, uncompetitive and non-competitive inhibitors. Biochem J. 1974 Jan;137(1):143–144. doi: 10.1042/bj1370143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIRHEIMER G., EBEL J. P. CARACT'ERISATION D'UNE POLYPHOSPHATE-AMP-PHOSPHOTRANSF'ERASE DANS CORYNEBACTERIUM XEROSIS. C R Hebd Seances Acad Sci. 1965 Mar 29;260:3787–3790. [PubMed] [Google Scholar]
- Dawes E. A., Senior P. J. The role and regulation of energy reserve polymers in micro-organisms. Adv Microb Physiol. 1973;10:135–266. doi: 10.1016/s0065-2911(08)60088-0. [DOI] [PubMed] [Google Scholar]
- Felter S., Stahl A. J. Enzymes du métabolisme des polyphosphates dans la levure. 3. Purification et propriétés de la polyphosphate-ADP-phosphotransferase. Biochimie. 1973;55(3):245–251. doi: 10.1016/s0300-9084(73)80122-1. [DOI] [PubMed] [Google Scholar]
- KORNBERG S. R. Adenosine triphosphate synthesis from polyphosphate by an enzyme from Escherichia coli. Biochim Biophys Acta. 1957 Nov;26(2):294–300. doi: 10.1016/0006-3002(57)90008-2. [DOI] [PubMed] [Google Scholar]
- Kulaev I. S., Shimona O., Bobyk M. A. O biosinteze neorganicheskikh polifosfatov u Neurospora crassa. Biokhimiia. 1968 May-Jun;33(3):419–434. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- MUHAMMED A., RODGERS A., HUGHES D. E. Purification and properties of a polymetaphosphatase from Corynebacterium xerosis. J Gen Microbiol. 1959 Jun;20(3):482–495. doi: 10.1099/00221287-20-3-482. [DOI] [PubMed] [Google Scholar]
- Pepin C. A., Wood H. G. Polyphosphate glucokinase from Propionibacterium shermanii. Kinetics and demonstration that the mechanism involves both processive and nonprocessive type reactions. J Biol Chem. 1986 Apr 5;261(10):4476–4480. [PubMed] [Google Scholar]
- Robinson N. A., Goss N. H., Wood H. G. Polyphosphate kinase from Propionibacterium shermanii: formation of an enzymatically active insoluble complex with basic proteins and characterization of synthesized polyphosphate. Biochem Int. 1984 Jun;8(6):757–769. [PubMed] [Google Scholar]
- Robinson N. A., Wood H. G. Polyphosphate kinase from Propionibacterium shermanii. Demonstration that the synthesis and utilization of polyphosphate is by a processive mechanism. J Biol Chem. 1986 Apr 5;261(10):4481–4485. [PubMed] [Google Scholar]
- SZYMONA M., OSTROWSKI W. INORGANIC POLYPHOSPHATE GLUCOKINASE OF MYCOBACTERIUM PHLEI. Biochim Biophys Acta. 1964 May 4;85:283–295. doi: 10.1016/0926-6569(64)90249-4. [DOI] [PubMed] [Google Scholar]
- Suzuki H., Kaneko T., Ikeda Y. Properties of polyphosphate kinase prepared from mycobacterium smegmatis. Biochim Biophys Acta. 1972 May 12;268(2):381–390. doi: 10.1016/0005-2744(72)90333-6. [DOI] [PubMed] [Google Scholar]
- Wood H. G., Clark J. E. Biological aspects of inorganic polyphosphates. Annu Rev Biochem. 1988;57:235–260. doi: 10.1146/annurev.bi.57.070188.001315. [DOI] [PubMed] [Google Scholar]
- van Groenestijn J. W., Bentvelsen M. M., Deinema M. H., Zehnder A. J. Polyphosphate-degrading enzymes in Acinetobacter spp. and activated sludge. Appl Environ Microbiol. 1989 Jan;55(1):219–223. doi: 10.1128/aem.55.1.219-223.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Groenestijn J. W., Zuidema M., van de Worp J. J., Deinema M. H., Zehnder A. J. Influence of environmental parameters on polyphosphate accumulation in Acinetobacter sp. Antonie Van Leeuwenhoek. 1989;55(1):67–82. doi: 10.1007/BF02309620. [DOI] [PubMed] [Google Scholar]