Abstract
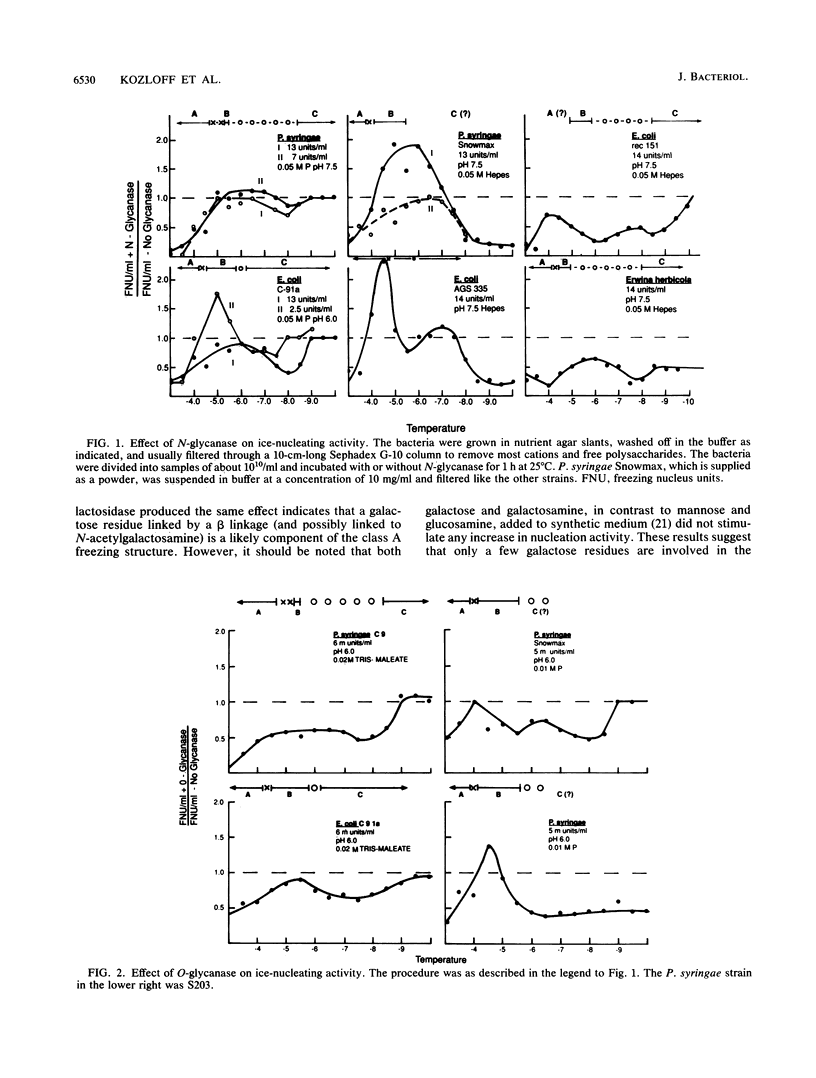
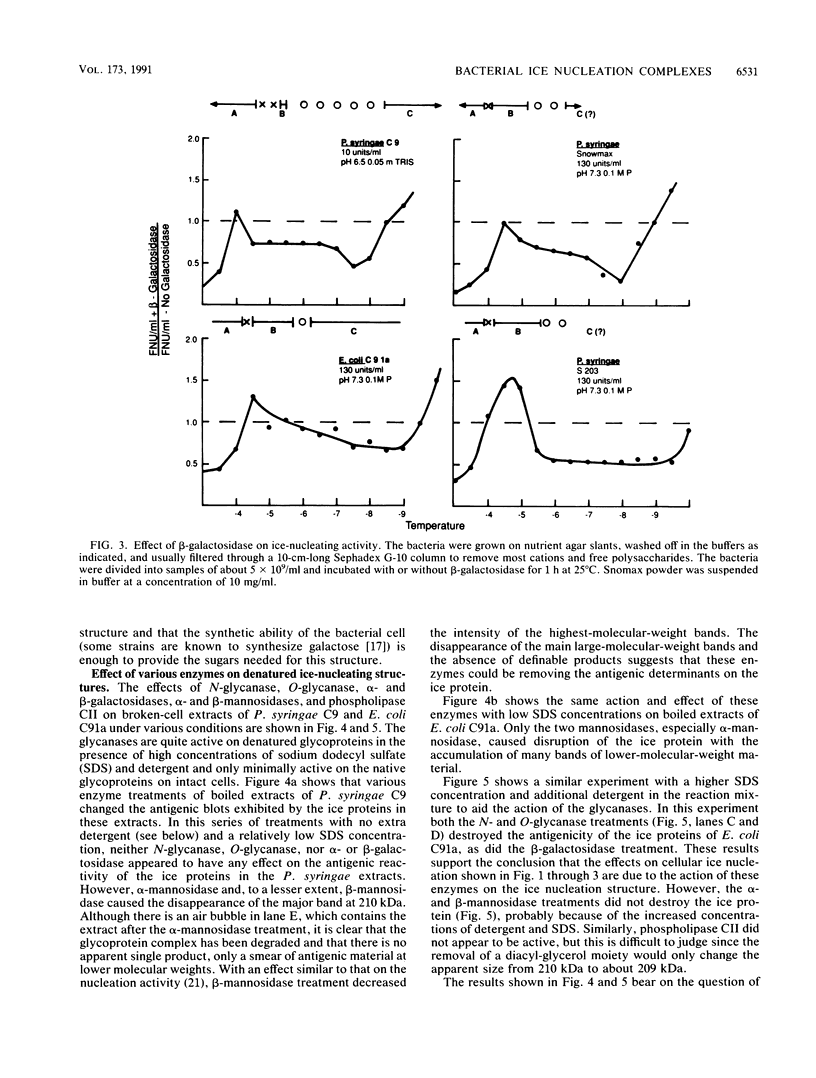
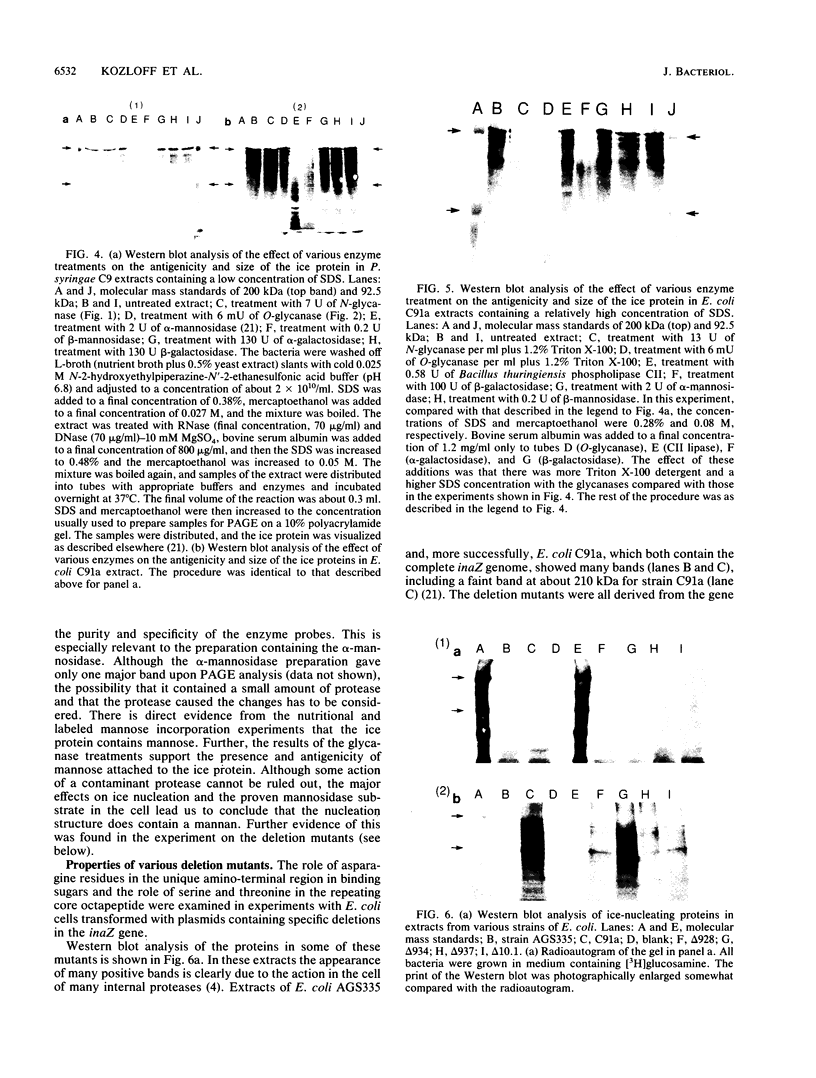
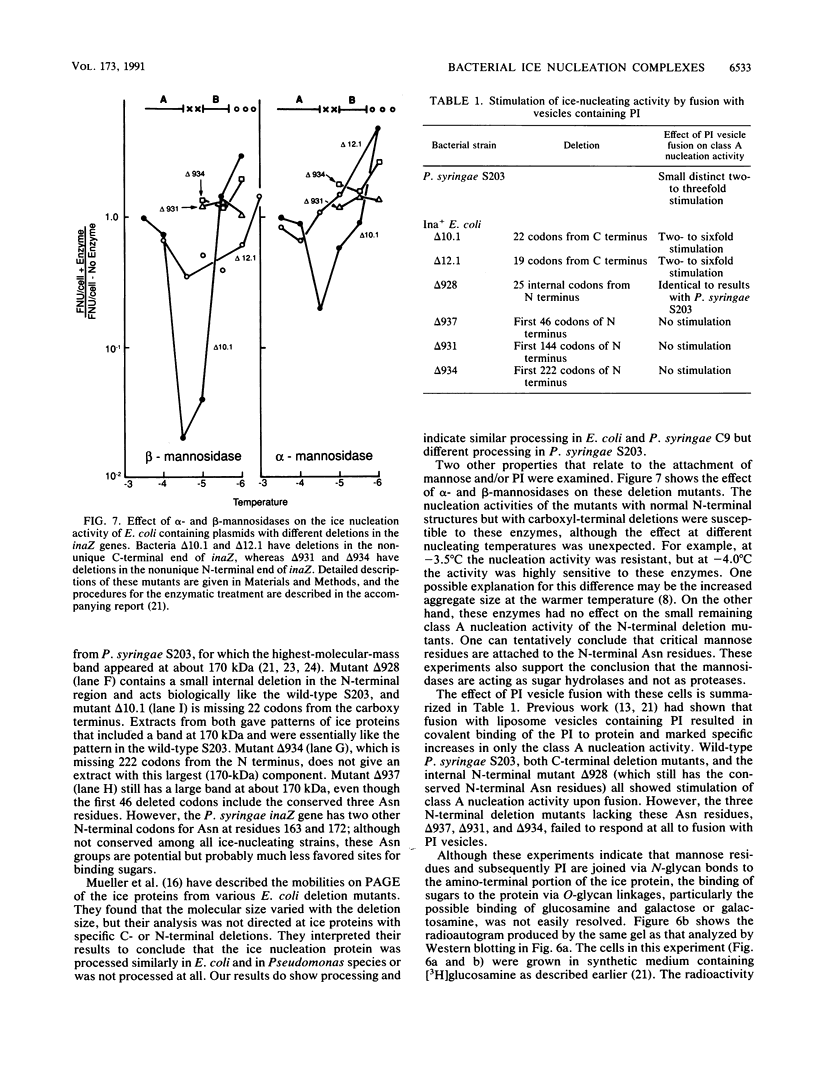
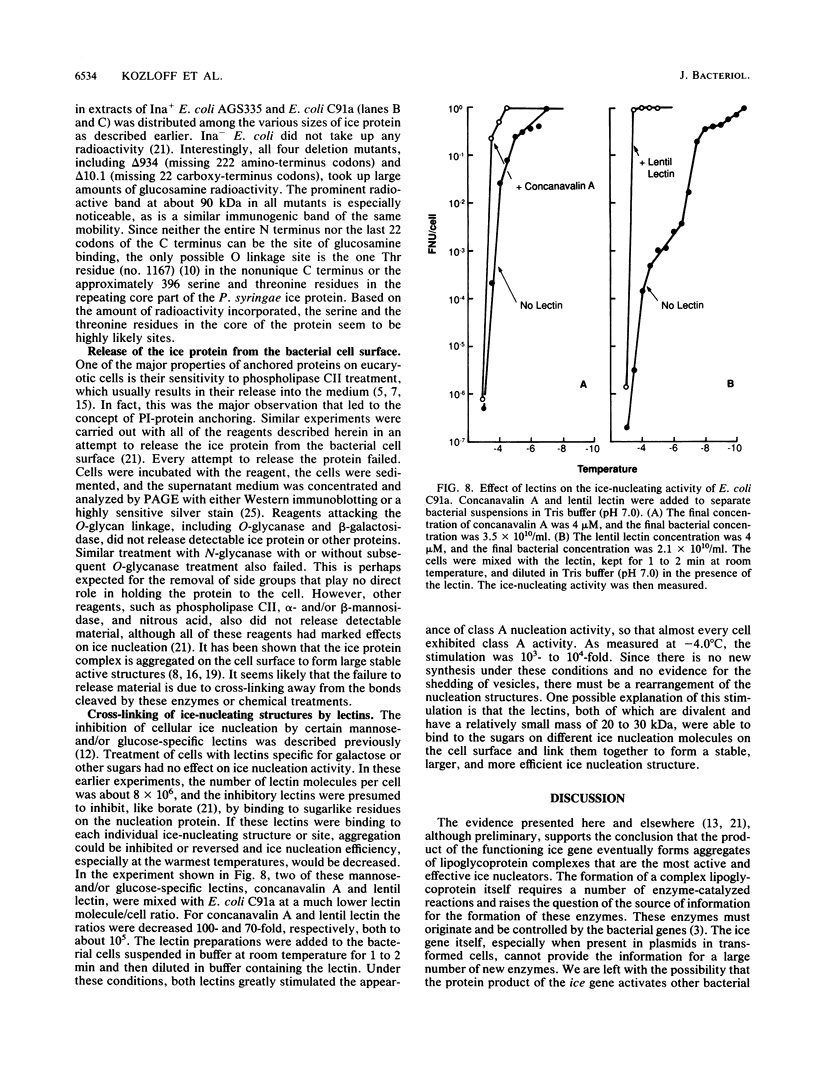
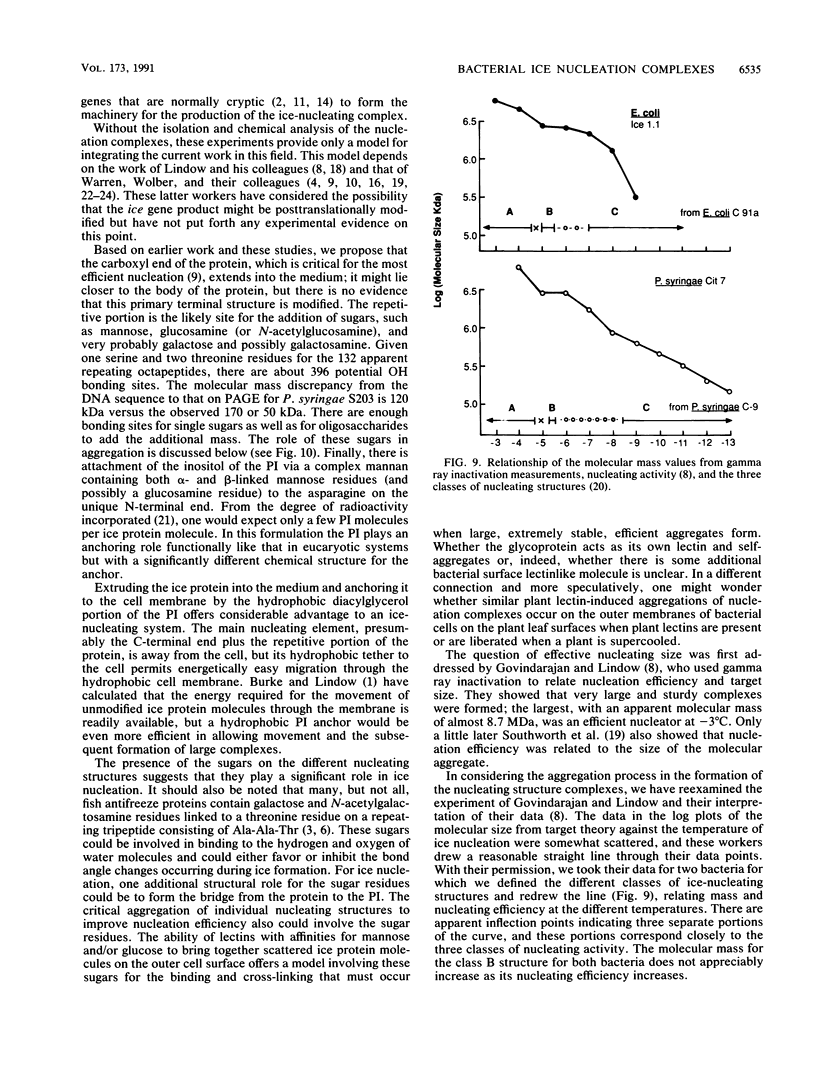
The preliminary finding that nonprotein additions to the protein product of the ice-nucleating gene of Pseudomonas syringae or Erwinia herbicola are essential for ice nucleation at the warmest temperatures has led to experiments aimed at identifying possible linkages between the ice protein and the other components. It appears that the protein is coupled to various sugars through N- and O-glycan linkages. Mannose residues are apparently bound via an N-glycan bond to the amide nitrogen of one or more of the three essential asparagine residues in the unique amino-terminal portion of the protein. In turn, these mannose residues are involved in the subsequent attachment of phosphatidylinositol to the nucleation structure. This phosphatidylinositol-mannose-protein structure is the critical element in the class A nucleating structure. In addition to sugars attached to the asparagine residues, additional sugar residues appear to be attached by O-glycan linkages to serine and threonine residues in the primary repeating octapeptide, which makes up 70% of the total ice protein. These additional sugar residues include galactose and glucosamine and most likely additional mannose residues. These conclusions were based on (i) the changes in ice-nucleating activity due to the action of N- and O-glycanases, alpha- and beta-mannosidoses, and beta-galactosidase; (ii) immunoblot analyses of ice proteins in cell extracts after enzyme treatments; and (iii) the properties of transformed Ice+ Escherichia coli cells containing plasmids with defined amino-terminal and carboxyl-terminal deletions in the ice gene. Finally, evidence is presented that these sugar residues may play a role in aggregating the ice gene lipoglycoprotein compound into larger aggregates, which are the most effective ice nucleation structures.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Davies P. L., Hew C. L. Biochemistry of fish antifreeze proteins. FASEB J. 1990 May;4(8):2460–2468. doi: 10.1096/fasebj.4.8.2185972. [DOI] [PubMed] [Google Scholar]
- Deininger C. A., Mueller G. M., Wolber P. K. Immunological characterization of ice nucleation proteins from Pseudomonas syringae, Pseudomonas fluorescens, and Erwinia herbicola. J Bacteriol. 1988 Feb;170(2):669–675. doi: 10.1128/jb.170.2.669-675.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doering T. L., Masterson W. J., Hart G. W., Englund P. T. Biosynthesis of glycosyl phosphatidylinositol membrane anchors. J Biol Chem. 1990 Jan 15;265(2):611–614. [PubMed] [Google Scholar]
- Feeney R. E., Burcham T. S., Yeh Y. Antifreeze glycoproteins from polar fish blood. Annu Rev Biophys Biophys Chem. 1986;15:59–78. doi: 10.1146/annurev.bb.15.060186.000423. [DOI] [PubMed] [Google Scholar]
- Ferguson M. A., Williams A. F. Cell-surface anchoring of proteins via glycosyl-phosphatidylinositol structures. Annu Rev Biochem. 1988;57:285–320. doi: 10.1146/annurev.bi.57.070188.001441. [DOI] [PubMed] [Google Scholar]
- Govindarajan A. G., Lindow S. E. Size of bacterial ice-nucleation sites measured in situ by radiation inactivation analysis. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1334–1338. doi: 10.1073/pnas.85.5.1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green R. L., Corotto L. V., Warren G. J. Deletion mutagenesis of the ice nucleation gene from Pseudomonas syringae S203. Mol Gen Genet. 1988 Dec;215(1):165–172. doi: 10.1007/BF00331320. [DOI] [PubMed] [Google Scholar]
- Hall B. G., Yokoyama S., Calhoun D. H. Role of cryptic genes in microbial evolution. Mol Biol Evol. 1983 Dec;1(1):109–124. doi: 10.1093/oxfordjournals.molbev.a040300. [DOI] [PubMed] [Google Scholar]
- Kozloff L. M., Lute M., Westaway D. Phosphatidylinositol as a Component of the Ice Nucleating Site of Pseudomonas syringae and Erwinia herbiola. Science. 1984 Nov 16;226(4676):845–846. doi: 10.1126/science.226.4676.845. [DOI] [PubMed] [Google Scholar]
- Kozloff L. M., Turner M. A., Arellano F., Lute M. Phosphatidylinositol, a phospholipid of ice-nucleating bacteria. J Bacteriol. 1991 Mar;173(6):2053–2060. doi: 10.1128/jb.173.6.2053-2060.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low M. G. Glycosyl-phosphatidylinositol: a versatile anchor for cell surface proteins. FASEB J. 1989 Mar;3(5):1600–1608. doi: 10.1096/fasebj.3.5.2522071. [DOI] [PubMed] [Google Scholar]
- Mueller G. M., Wolber P. K., Warren G. J. Clustering of ice nucleation protein correlates with ice nucleation activity. Cryobiology. 1990 Aug;27(4):416–422. doi: 10.1016/0011-2240(90)90018-y. [DOI] [PubMed] [Google Scholar]
- Southworth M. W., Wolber P. K., Warren G. J. Nonlinear relationship between concentration and activity of a bacterial ice nucleation protein. J Biol Chem. 1988 Oct 15;263(29):15211–15216. [PubMed] [Google Scholar]
- Turner M. A., Arellano F., Kozloff L. M. Components of ice nucleation structures of bacteria. J Bacteriol. 1991 Oct;173(20):6515–6527. doi: 10.1128/jb.173.20.6515-6527.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner M. A., Arellano F., Kozloff L. M. Three separate classes of bacterial ice nucleation structures. J Bacteriol. 1990 May;172(5):2521–2526. doi: 10.1128/jb.172.5.2521-2526.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren G., Corotto L. The consensus sequence of ice nucleation proteins from Erwinia herbicola, Pseudomonas fluorescens and Pseudomonas syringae. Gene. 1989 Dec 21;85(1):239–242. doi: 10.1016/0378-1119(89)90488-5. [DOI] [PubMed] [Google Scholar]
- Wolber P. K., Deininger C. A., Southworth M. W., Vandekerckhove J., van Montagu M., Warren G. J. Identification and purification of a bacterial ice-nucleation protein. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7256–7260. doi: 10.1073/pnas.83.19.7256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolber P., Warren G. Bacterial ice-nucleation proteins. Trends Biochem Sci. 1989 May;14(5):179–182. doi: 10.1016/0968-0004(89)90270-3. [DOI] [PubMed] [Google Scholar]
- Wray W., Boulikas T., Wray V. P., Hancock R. Silver staining of proteins in polyacrylamide gels. Anal Biochem. 1981 Nov 15;118(1):197–203. doi: 10.1016/0003-2697(81)90179-2. [DOI] [PubMed] [Google Scholar]