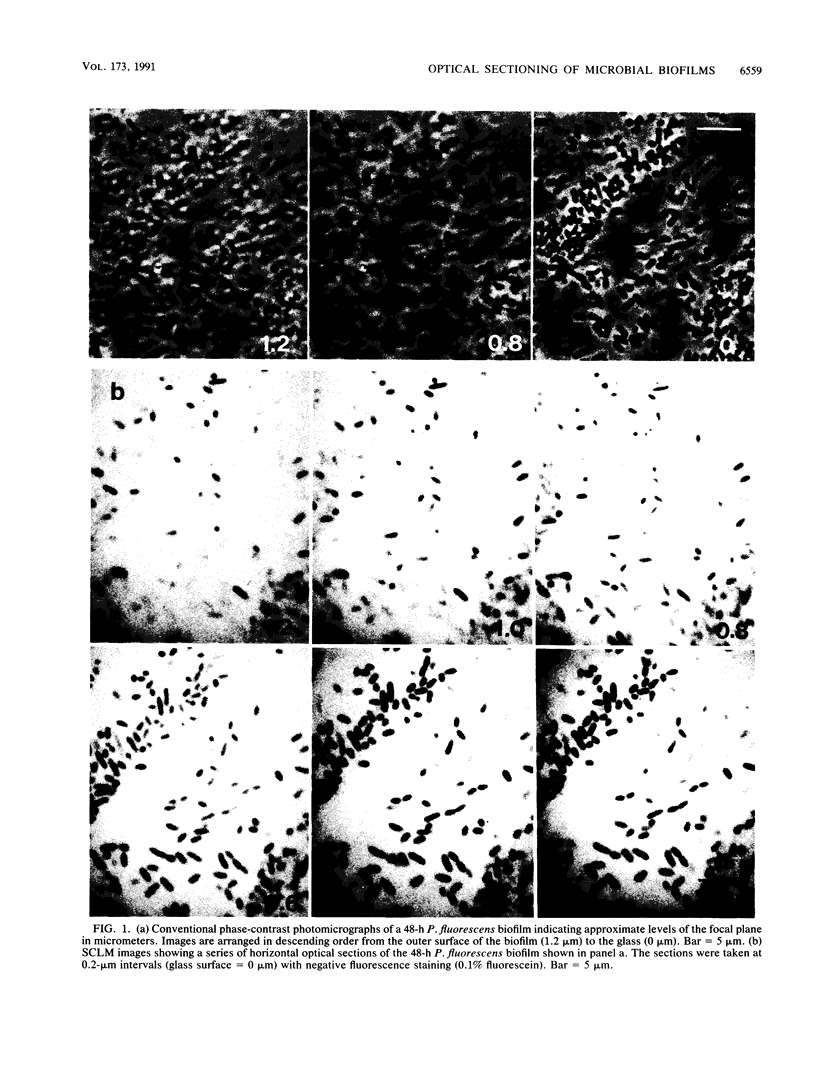
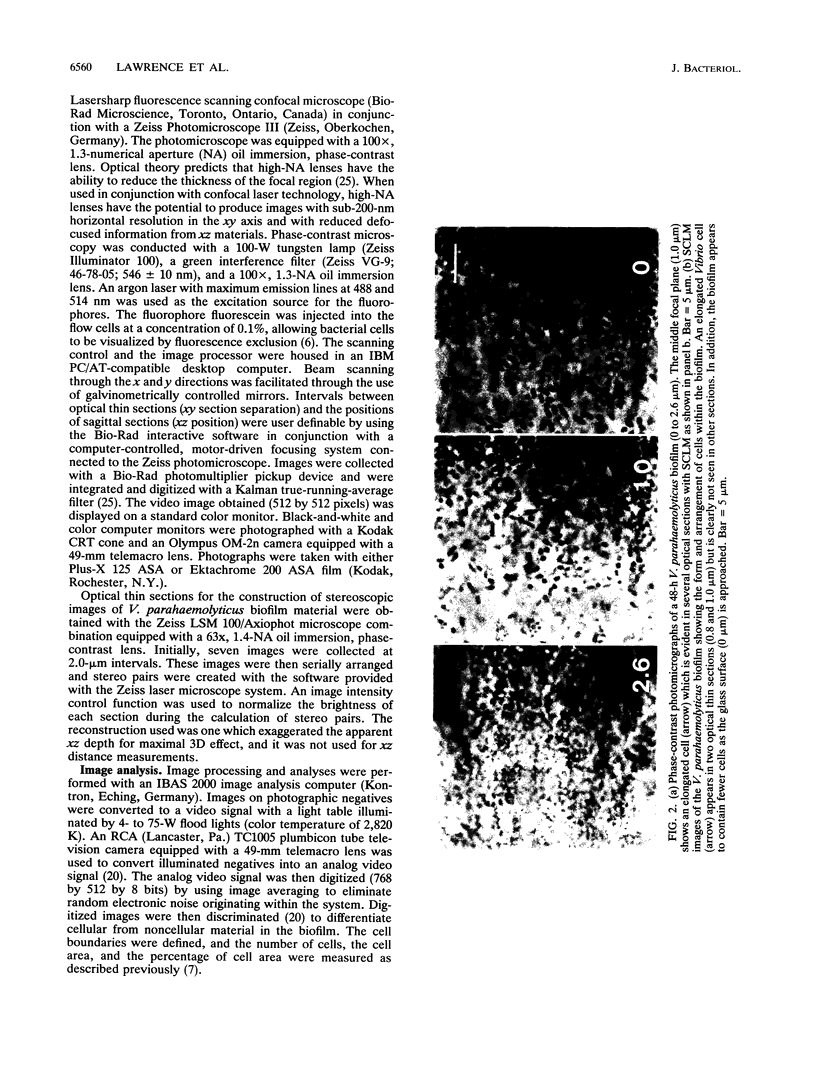
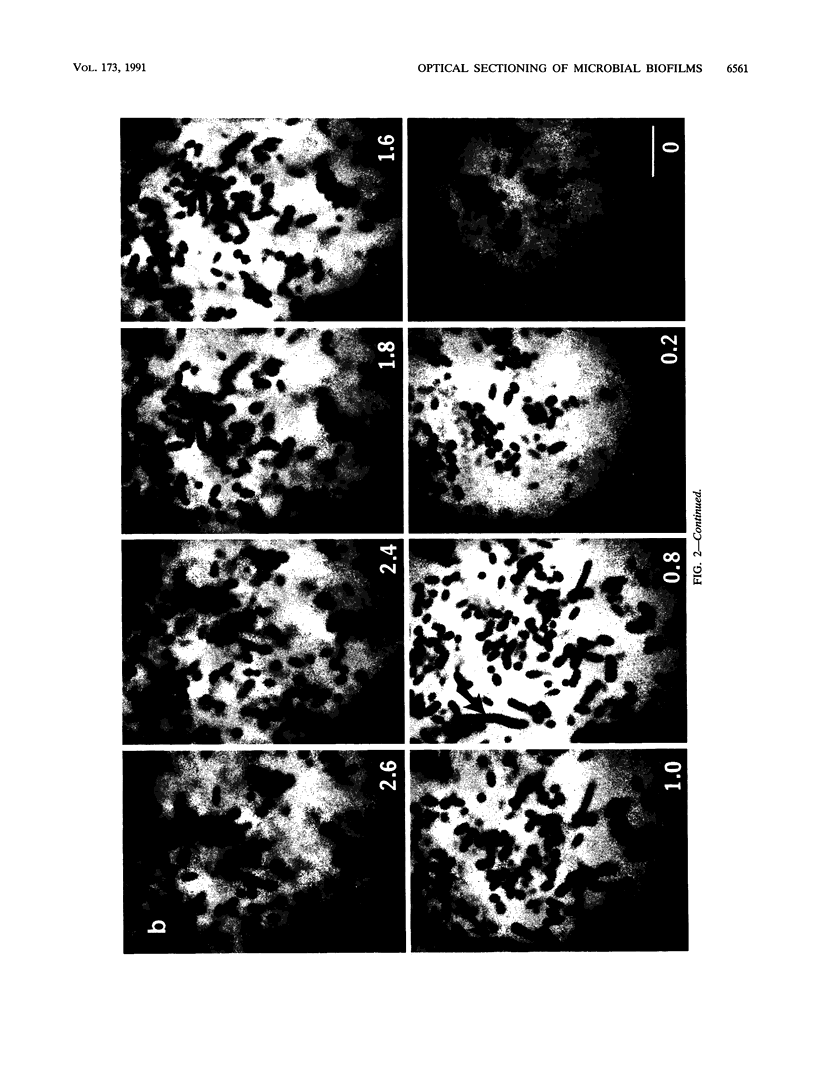
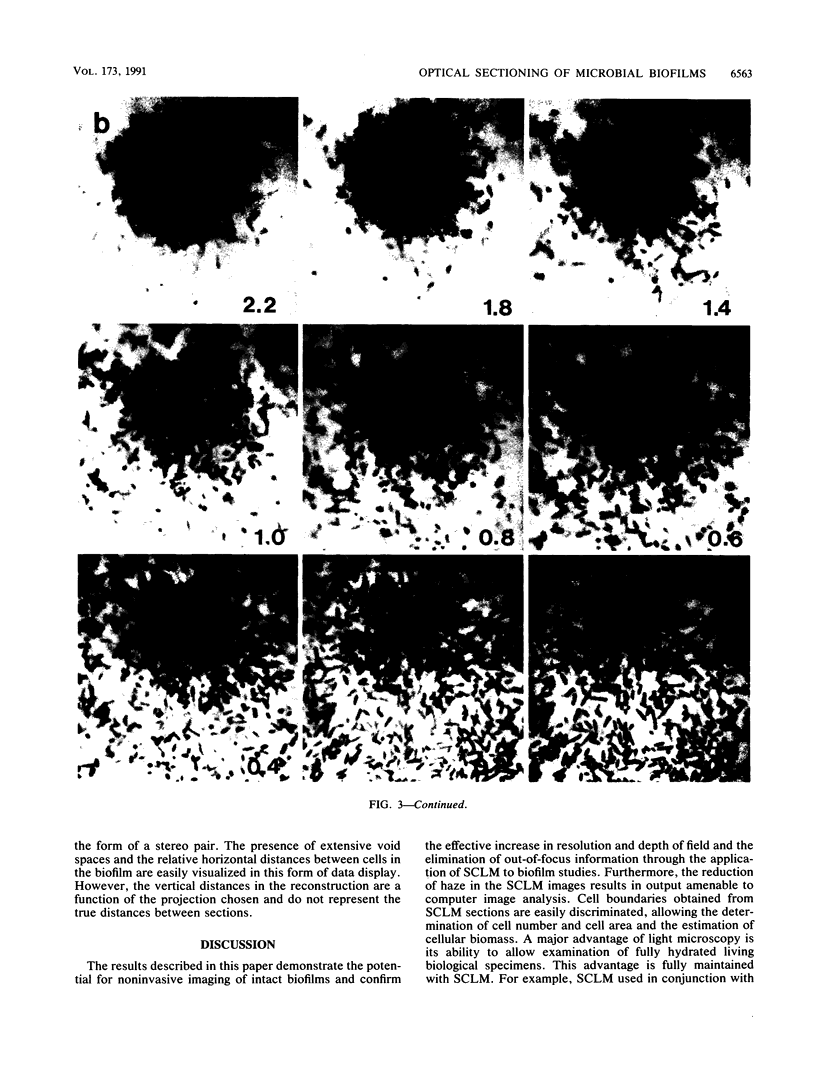
Abstract
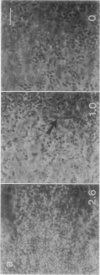
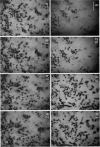
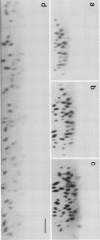
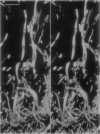
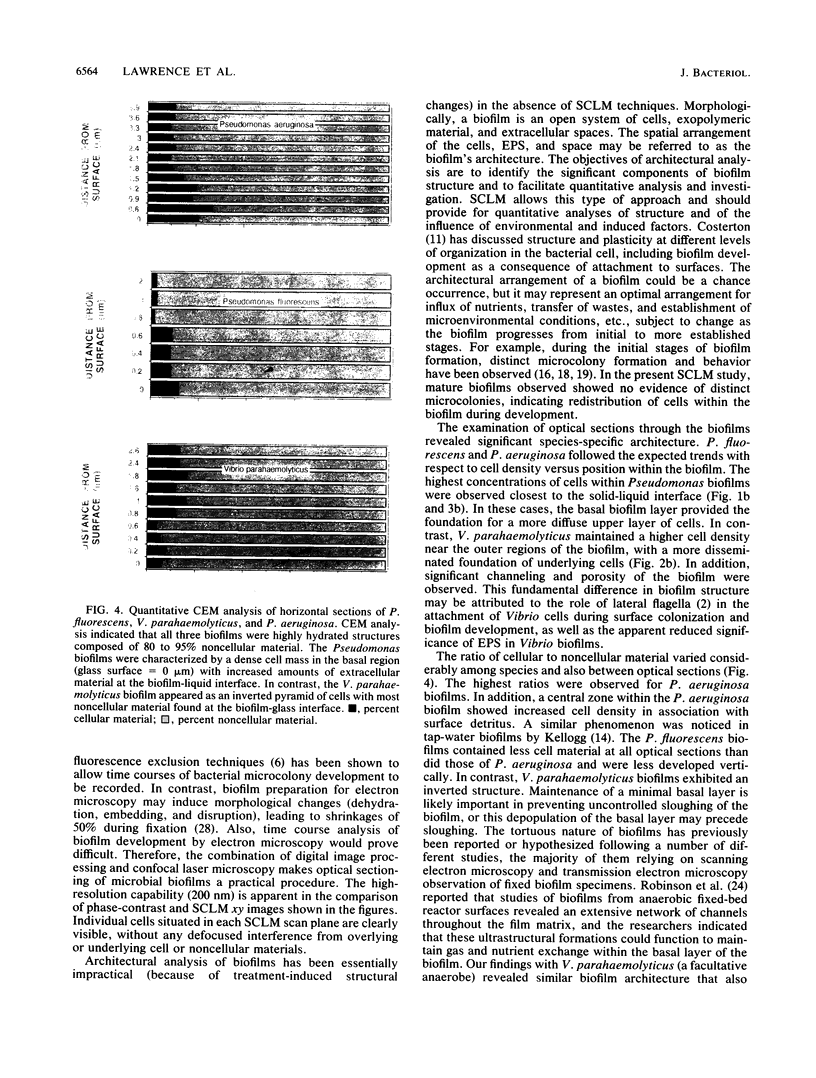
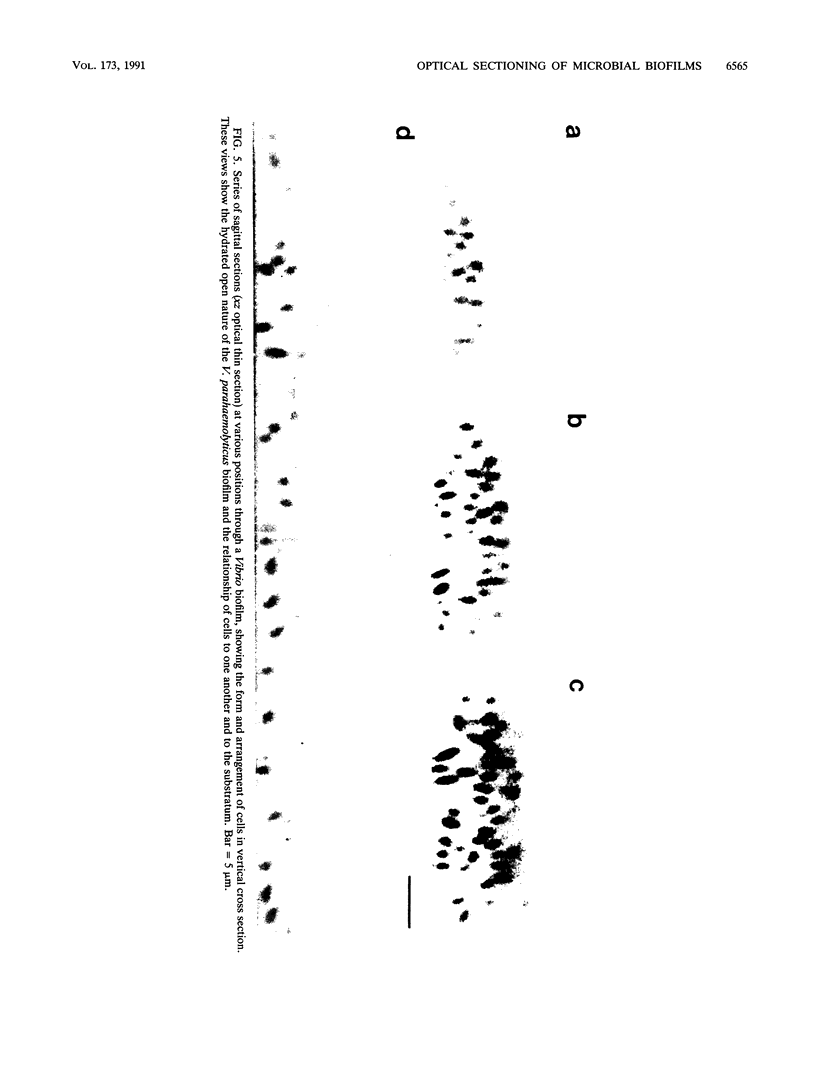
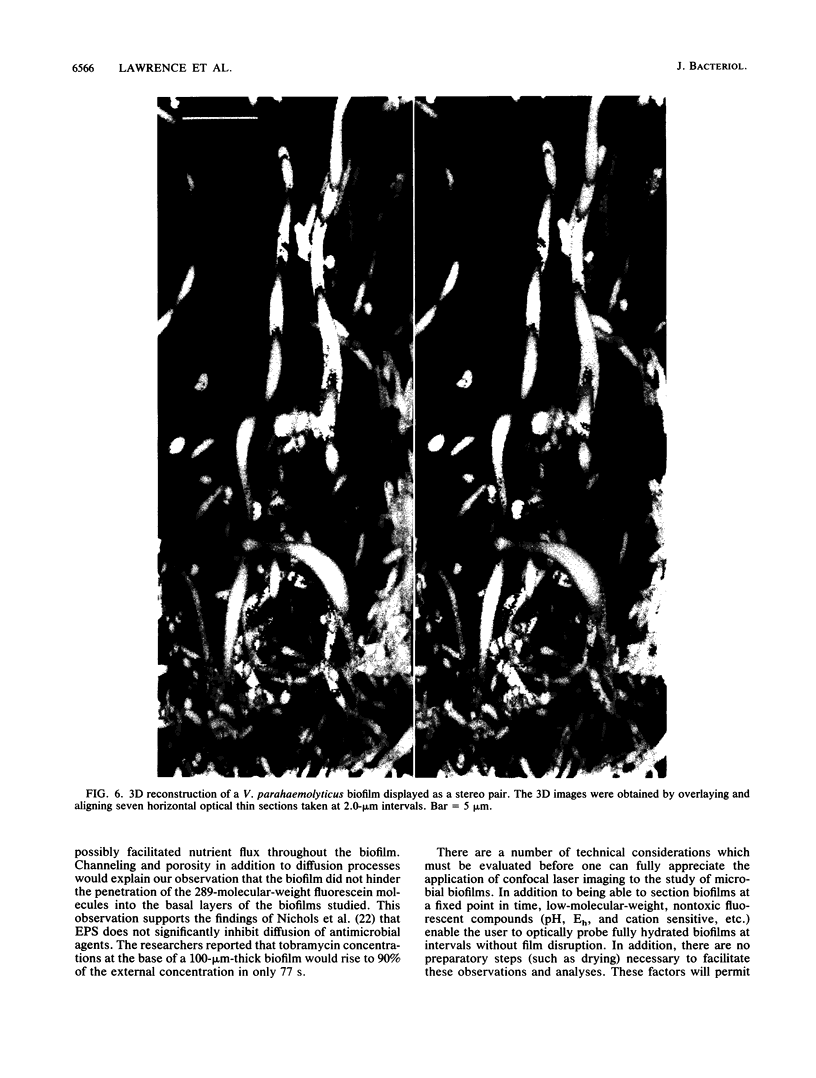
Scanning confocal laser microscopy (SCLM) was used to visualize fully hydrated microbial biofilms. The improved rejection of out-of-focus haze and the increased resolution of SCLM made it preferable to conventional phase microscopy for the analysis of living biofilms. The extent of image improvement was dependent on the characteristics of individual biofilms and was most apparent when films were dispersed in three dimensions, when they were thick, and when they contained a high number of cells. SCLM optical sections were amenable to quantitative computer-enhanced microscopy analyses, with minimal interference originating from overlying or underlying cell material. By using SCLM in conjunction with viable negative fluorescence staining techniques, horizontal (xy) and sagittal (xz) sections of intact biofilms of Pseudomonas aeruginosa, Pseudomonas fluorescens, and Vibrio parahaemolyticus were obtained. These optical sections were then analyzed by image-processing techniques to assess the distribution of cellular and noncellular areas within the biofilm matrices. The Pseudomonas biofilms were most cell dense at their attachment surfaces and became increasingly diffuse near their outer regions, whereas the Vibrio biofilms exhibited the opposite trend. Biofilms consisting of different species exhibited distinctive arrangements of the major biofilm structural components (cellular and extracellular materials and space). In general, biofilms were found to be highly hydrated, open structures composed of 73 to 98% extracellular materials and space. The use of xz sectioning revealed more detail of biofilm structure, including the presence of large void spaces within the Vibrio biofilms. In addition, three-dimensional reconstructions of biofilms were constructed and were displayed as stereo pairs. Application of the concepts of architectural analysis to mixed- or pure-species biofilms will allow detailed examination of the relationships among biofilm structure, adaptation, and response to stress.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agard D. A., Hiraoka Y., Shaw P., Sedat J. W. Fluorescence microscopy in three dimensions. Methods Cell Biol. 1989;30:353–377. doi: 10.1016/s0091-679x(08)60986-3. [DOI] [PubMed] [Google Scholar]
- Belas M. R., Colwell R. R. Adsorption kinetics of laterally and polarly flagellated Vibrio. J Bacteriol. 1982 Sep;151(3):1568–1580. doi: 10.1128/jb.151.3.1568-1580.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belas R., Simon M., Silverman M. Regulation of lateral flagella gene transcription in Vibrio parahaemolyticus. J Bacteriol. 1986 Jul;167(1):210–218. doi: 10.1128/jb.167.1.210-218.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brakenhoff G. J., van der Voort H. T., Baarslag M. W., Mans B., Oud J. L., Zwart R., van Driel R. Visualization and analysis techniques for three dimensional information acquired by confocal microscopy. Scanning Microsc. 1988 Dec;2(4):1831–1838. [PubMed] [Google Scholar]
- Carlsson K., Liljeborg A. A confocal laser microscope scanner for digital recording of optical serial sections. J Microsc. 1989 Feb;153(Pt 2):171–180. doi: 10.1111/j.1365-2818.1989.tb00557.x. [DOI] [PubMed] [Google Scholar]
- Carlsson K., Wallén P., Brodin L. Three-dimensional imaging of neurons by confocal fluorescence microscopy. J Microsc. 1989 Jul;155(Pt 1):15–26. doi: 10.1111/j.1365-2818.1989.tb04296.x. [DOI] [PubMed] [Google Scholar]
- Costerton J. W., Cheng K. J., Geesey G. G., Ladd T. I., Nickel J. C., Dasgupta M., Marrie T. J. Bacterial biofilms in nature and disease. Annu Rev Microbiol. 1987;41:435–464. doi: 10.1146/annurev.mi.41.100187.002251. [DOI] [PubMed] [Google Scholar]
- Costerton J. W. Structure and plasticity at various organization levels in the bacterial cell. Can J Microbiol. 1988 Apr;34(4):513–521. doi: 10.1139/m88-088. [DOI] [PubMed] [Google Scholar]
- Eighmy T. T., Maratea D., Bishop P. L. Electron microscopic examination of wastewater biofilm formation and structural components. Appl Environ Microbiol. 1983 Jun;45(6):1921–1931. doi: 10.1128/aem.45.6.1921-1931.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinner N. E., Balkwill D. L., Bishop P. L. Light and electron microscopic studies of microorganisms growing in rotating biological contactor biofilms. Appl Environ Microbiol. 1983 May;45(5):1659–1669. doi: 10.1128/aem.45.5.1659-1669.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols W. W., Dorrington S. M., Slack M. P., Walmsley H. L. Inhibition of tobramycin diffusion by binding to alginate. Antimicrob Agents Chemother. 1988 Apr;32(4):518–523. doi: 10.1128/aac.32.4.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson R. W., Akin D. E., Nordstedt R. A., Thomas M. V., Aldrich H. C. Light and electron microscopic examinations of methane-producing biofilms from anaerobic fixed-bed reactors. Appl Environ Microbiol. 1984 Jul;48(1):127–136. doi: 10.1128/aem.48.1.127-136.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shotton D., White N. Confocal scanning microscopy: three-dimensional biological imaging. Trends Biochem Sci. 1989 Nov;14(11):435–439. doi: 10.1016/0968-0004(89)90096-0. [DOI] [PubMed] [Google Scholar]
- Woldringh C. L., de Jong M. A., van den Berg W., Koppes L. Morphological analysis of the division cycle of two Escherichia coli substrains during slow growth. J Bacteriol. 1977 Jul;131(1):270–279. doi: 10.1128/jb.131.1.270-279.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]