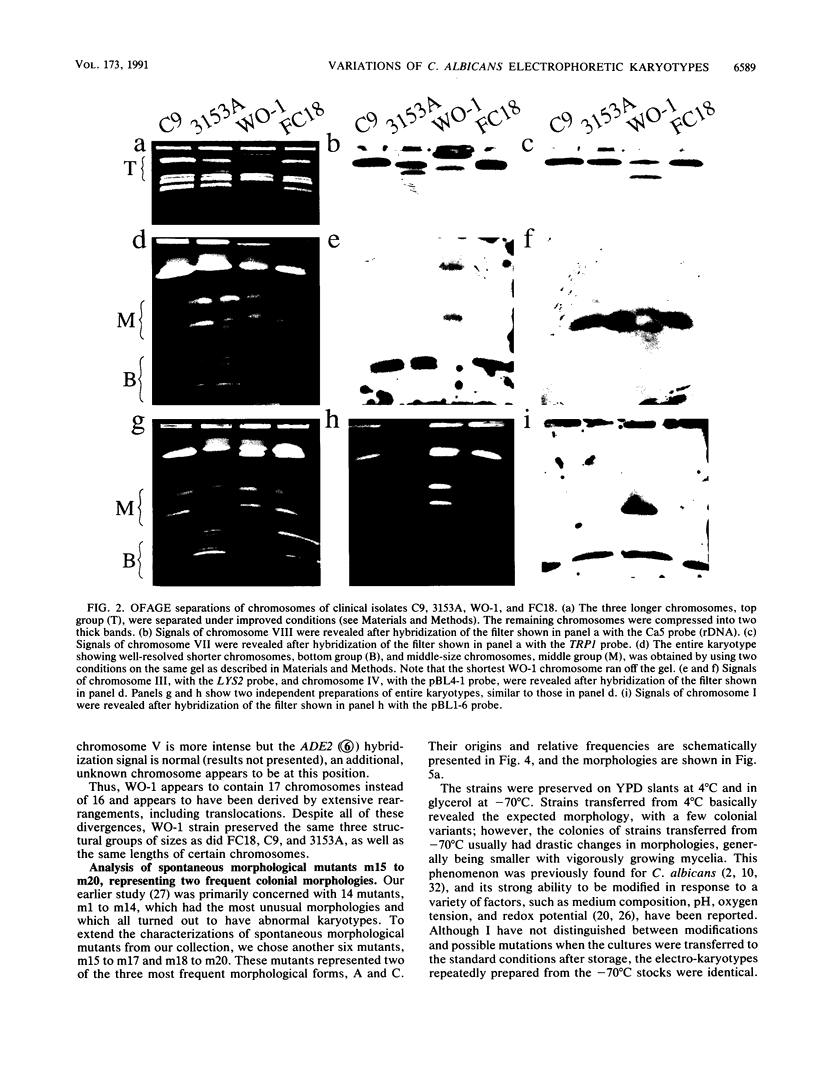
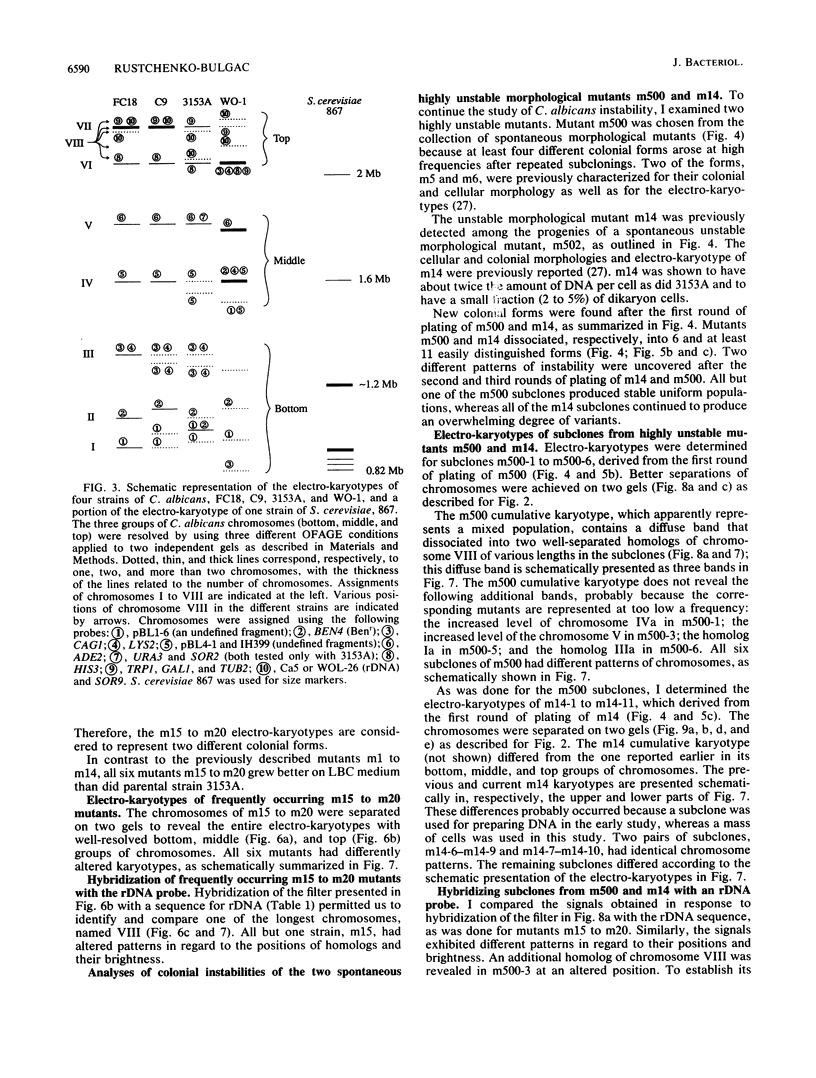
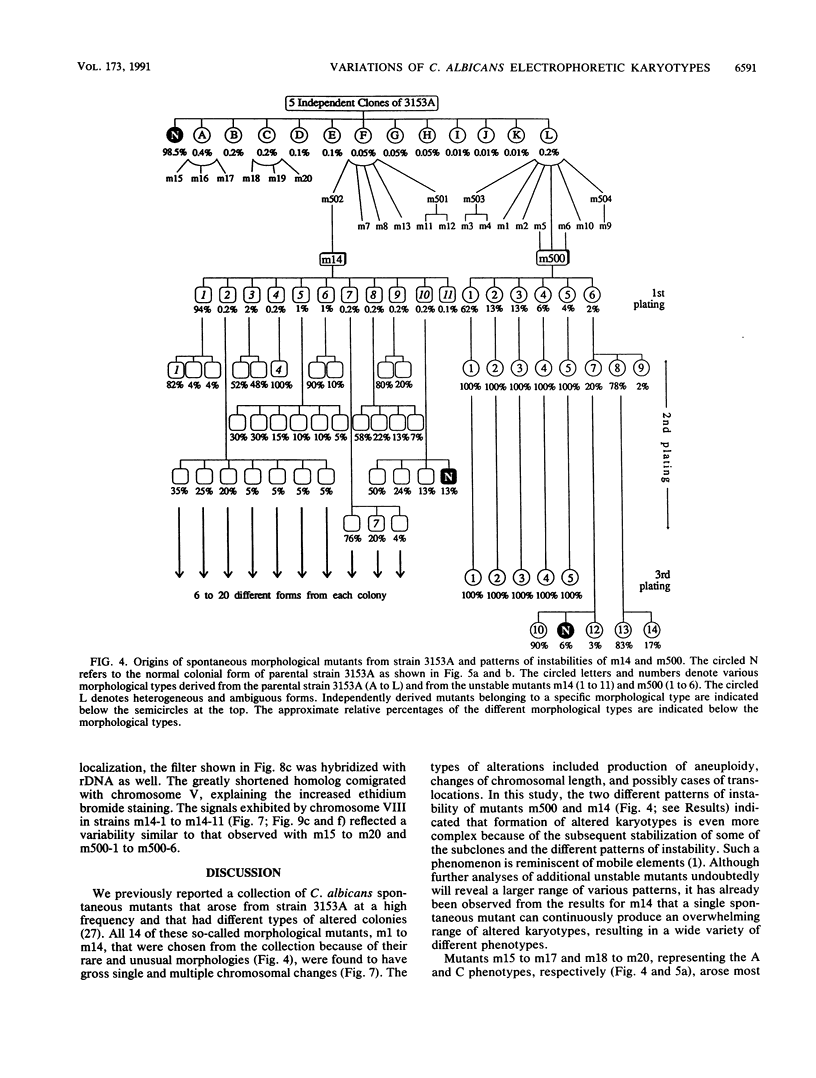
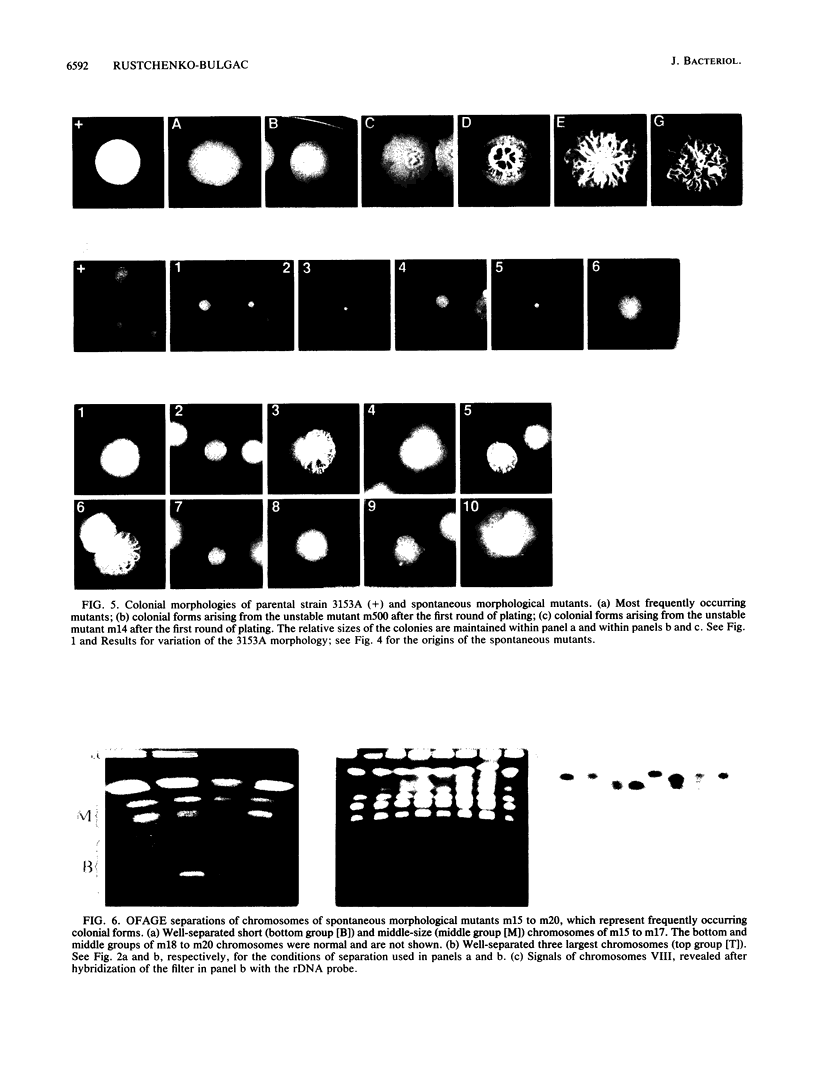
Abstract
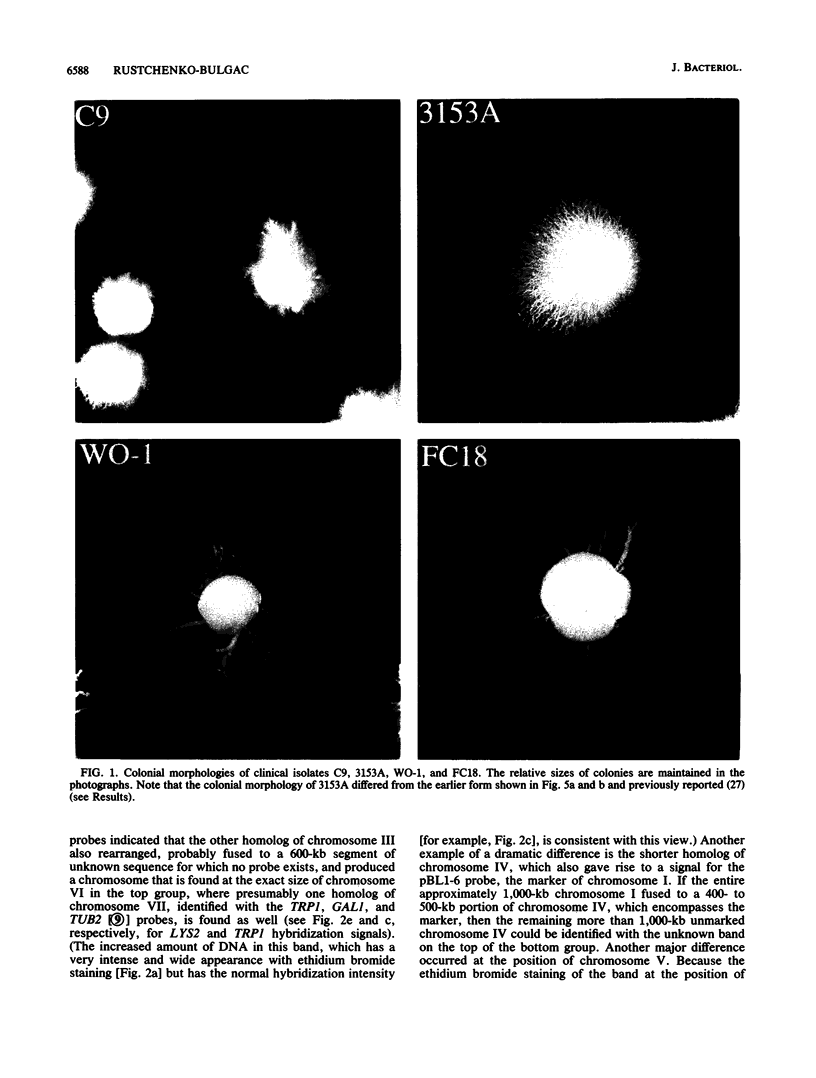
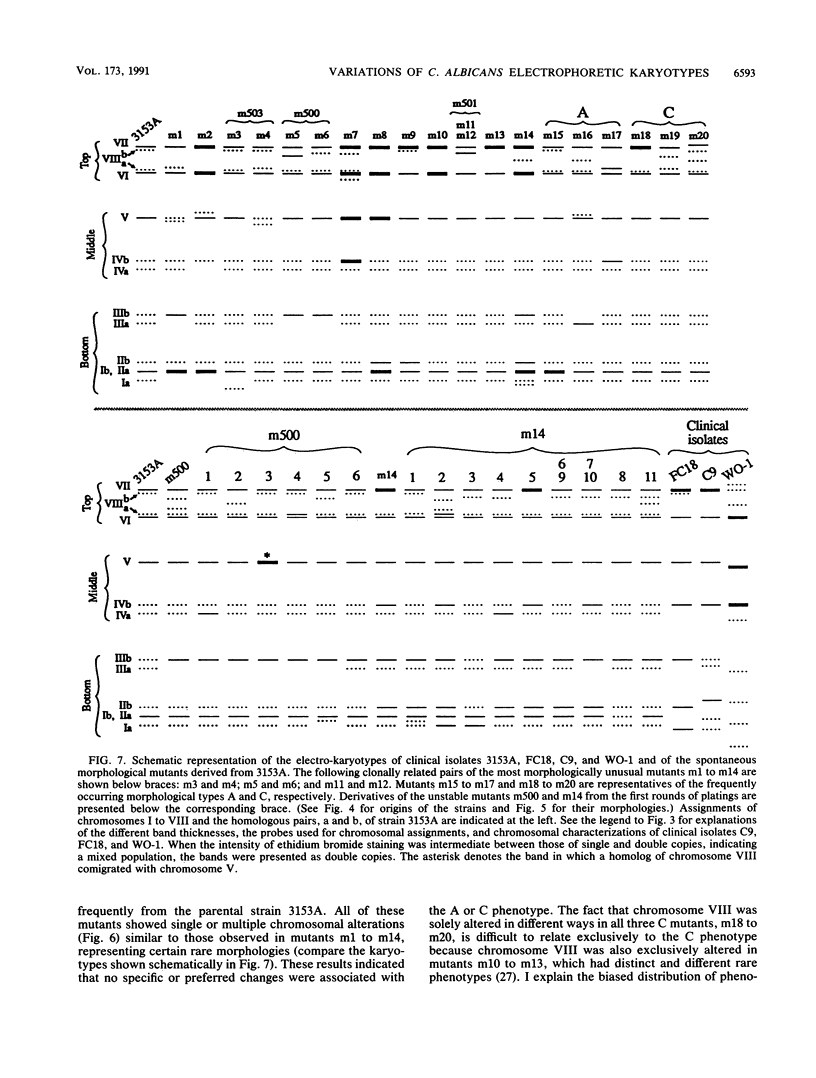
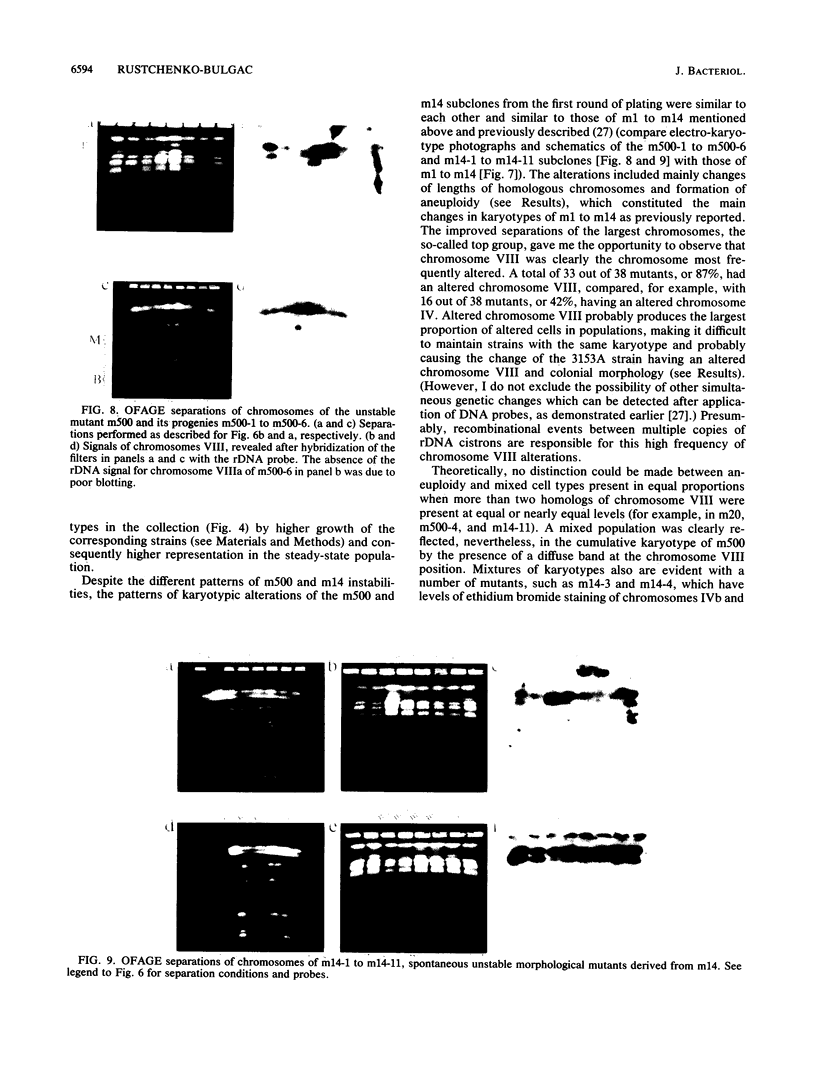
We previously described 14 rare spontaneous morphological mutants of Candida albicans that were associated with chromosomal aberrations (E. P. Rustchenko-Bulgac, F. Sherman, and J. B. Hicks, J. Bacteriol. 172:1276-1283, 1990). Improved conditions for separation of chromosomes, as well as hybridization probes, were used to investigate the variation of karyotypes of clinical isolates and additional morphological mutants. All 23 newly analyzed morphological mutants, representing frequently occurring and highly unstable colonial forms, had a variety of altered karyotypes. All chromosomal changes were similar to those previously observed in mutants m1 to m14. In this study, I particularly noted that the most frequent changes involved the long chromosome VIII, which carries ribosomal DNA cistrons. Two rates of instability were uncovered by analyzing the progenies from two highly unstable mutants. An unstable mutant proved to be able to continuously produce a large number of altered karyotypes that could result in a wide variety of different phenotypes. Furthermore, all four independent clinical isolates, FC18, C9, 3153A, and WO-1, common laboratory strains, revealed different electrophoretic karyotypes and distinct colonial morphologies on a synthetic medium, similar to spontaneous mutants. The differences of electrophoretic karyotypes observed among clinical isolates resembled the changes found among different kinds of spontaneous morphological mutants. These findings contribute to the understanding of natural karyotypic variability and are in agreement with the hypothesis that chromosomal alterations observed spontaneously under laboratory conditions provide this amictic species with genetic variability in nature.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carle G. F., Olson M. V. An electrophoretic karyotype for yeast. Proc Natl Acad Sci U S A. 1985 Jun;82(11):3756–3760. doi: 10.1073/pnas.82.11.3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carle G. F., Olson M. V. Separation of chromosomal DNA molecules from yeast by orthogonal-field-alternation gel electrophoresis. Nucleic Acids Res. 1984 Jul 25;12(14):5647–5664. doi: 10.1093/nar/12.14.5647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DI MENNA M. E. Natural occurrence of rough variant of a yeast, Candida albicans. Nature. 1952 Mar 29;169(4300):550–551. doi: 10.1038/169550b0. [DOI] [PubMed] [Google Scholar]
- Evans E. G., Odds F. C., Richardson M. D., Holland K. T. The effect of growth medium of filament production in Candida albicans. Sabouraudia. 1974 Mar;12(1):112–119. doi: 10.1080/00362177485380151. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Gillum A. M., Tsay E. Y., Kirsch D. R. Isolation of the Candida albicans gene for orotidine-5'-phosphate decarboxylase by complementation of S. cerevisiae ura3 and E. coli pyrF mutations. Mol Gen Genet. 1984;198(2):179–182. doi: 10.1007/BF00328721. [DOI] [PubMed] [Google Scholar]
- Iwaguchi S., Homma M., Tanaka K. Variation in the electrophoretic karyotype analysed by the assignment of DNA probes in Candida albicans. J Gen Microbiol. 1990 Dec;136(12):2433–2442. doi: 10.1099/00221287-136-12-2433. [DOI] [PubMed] [Google Scholar]
- Kurtz M. B., Cortelyou M. W., Kirsch D. R. Integrative transformation of Candida albicans, using a cloned Candida ADE2 gene. Mol Cell Biol. 1986 Jan;6(1):142–149. doi: 10.1128/mcb.6.1.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon-Chung K. J., Lehman D., Good C., Magee P. T. Genetic evidence for role of extracellular proteinase in virulence of Candida albicans. Infect Immun. 1985 Sep;49(3):571–575. doi: 10.1128/iai.49.3.571-575.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasker B. A., Carle G. F., Kobayashi G. S., Medoff G. Comparison of the separation of Candida albicans chromosome-sized DNA by pulsed-field gel electrophoresis techniques. Nucleic Acids Res. 1989 May 25;17(10):3783–3793. doi: 10.1093/nar/17.10.3783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. L., Buckley H. R., Campbell C. C. An amino acid liquid synthetic medium for the development of mycelial and yeast forms of Candida Albicans. Sabouraudia. 1975 Jul;13(2):148–153. doi: 10.1080/00362177585190271. [DOI] [PubMed] [Google Scholar]
- Magee B. B., Koltin Y., Gorman J. A., Magee P. T. Assignment of cloned genes to the seven electrophoretically separated Candida albicans chromosomes. Mol Cell Biol. 1988 Nov;8(11):4721–4726. doi: 10.1128/mcb.8.11.4721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magee B. B., Magee P. T. Electrophoretic karyotypes and chromosome numbers in Candida species. J Gen Microbiol. 1987 Feb;133(2):425–430. doi: 10.1099/00221287-133-2-425. [DOI] [PubMed] [Google Scholar]
- Mahrous M., Lott T. J., Meyer S. A., Sawant A. D., Ahearn D. G. Electrophoretic karyotyping of typical and atypical Candida albicans. J Clin Microbiol. 1990 May;28(5):876–881. doi: 10.1128/jcm.28.5.876-881.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merz W. G., Connelly C., Hieter P. Variation of electrophoretic karyotypes among clinical isolates of Candida albicans. J Clin Microbiol. 1988 May;26(5):842–845. doi: 10.1128/jcm.26.5.842-845.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortimer R. K., Schild D. Genetic map of Saccharomyces cerevisiae, edition 9. Microbiol Rev. 1985 Sep;49(3):181–213. doi: 10.1128/mr.49.3.181-213.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbluh A., Mevarech M., Koltin Y., Gorman J. A. Isolation of genes from Candida albicans by complementation in Saccharomyces cerevisiae. Mol Gen Genet. 1985;200(3):500–502. doi: 10.1007/BF00425739. [DOI] [PubMed] [Google Scholar]
- Rustchenko-Bulgac E. P., Sherman F., Hicks J. B. Chromosomal rearrangements associated with morphological mutants provide a means for genetic variation of Candida albicans. J Bacteriol. 1990 Mar;172(3):1276–1283. doi: 10.1128/jb.172.3.1276-1283.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadhu C., McEachern M. J., Rustchenko-Bulgac E. P., Schmid J., Soll D. R., Hicks J. B. Telomeric and dispersed repeat sequences in Candida yeasts and their use in strain identification. J Bacteriol. 1991 Jan;173(2):842–850. doi: 10.1128/jb.173.2.842-850.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slutsky B., Staebell M., Anderson J., Risen L., Pfaller M., Soll D. R. "White-opaque transition": a second high-frequency switching system in Candida albicans. J Bacteriol. 1987 Jan;169(1):189–197. doi: 10.1128/jb.169.1.189-197.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soll D. R., Langtimm C. J., McDowell J., Hicks J., Galask R. High-frequency switching in Candida strains isolated from vaginitis patients. J Clin Microbiol. 1987 Sep;25(9):1611–1622. doi: 10.1128/jcm.25.9.1611-1622.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel R. A., Sponcler R. S. The study and significance of colony dissociation in Candida albicans. Sabouraudia. 1970 Feb;7(4):273–278. doi: 10.1080/00362177085190511. [DOI] [PubMed] [Google Scholar]
- Whelan W. L., Partridge R. M., Magee P. T. Heterozygosity and segregation in Candida albicans. Mol Gen Genet. 1980;180(1):107–113. doi: 10.1007/BF00267358. [DOI] [PubMed] [Google Scholar]