Abstract
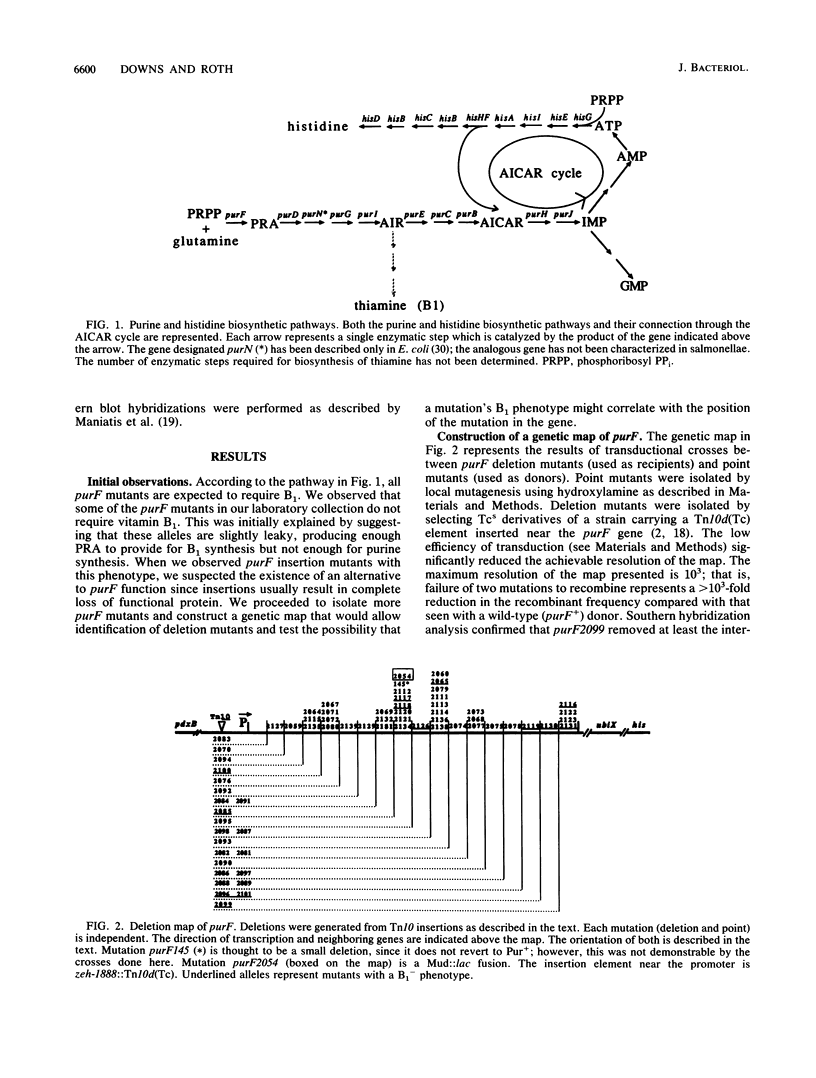
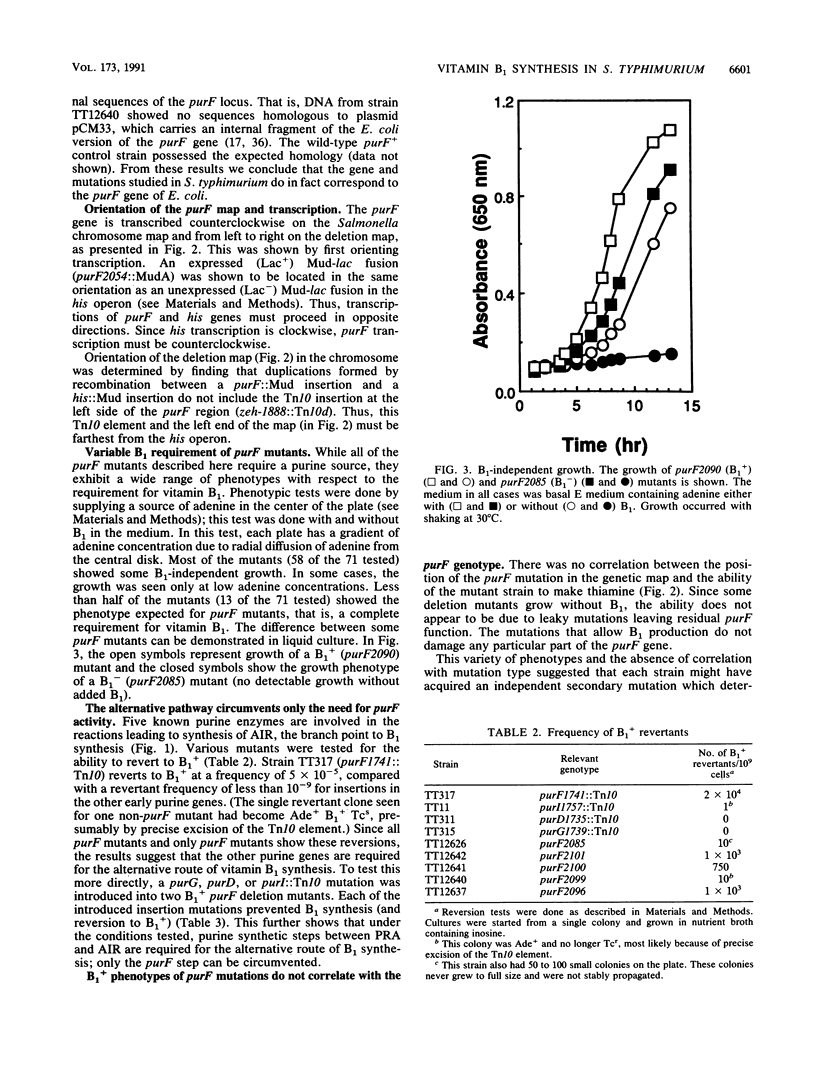
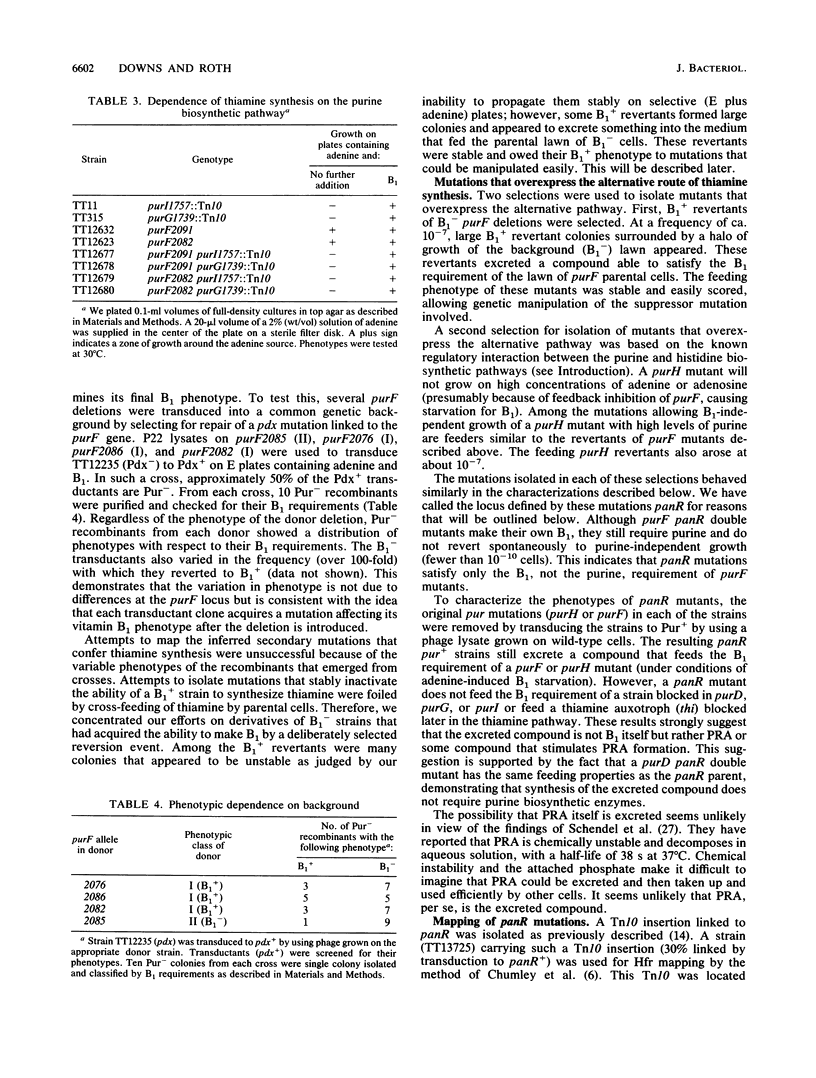
In Salmonella typhimurium, the first five steps in purine biosynthesis also serve as the first steps in the biosynthesis of the pyrimidine moiety of thiamine (vitamin B1). Strains with null mutations of the first gene of purine-thiamine synthesis (purF) can, under some circumstances, grow without thiamine. This suggests the existence of an alternative pathway to thiamine that can function without the purF protein. To demonstrate the nature and map position of the purF mutations corrected, a fine-structure genetic map of the purF gene was made. The map allows identification of deletion mutations that remove virtually all of the purF gene, as defined by mutations. We describe conditions and mutations (panR) which allow B1 synthesis appears to require enzymes which act mutants lacking purF function. The alternative route of B1 synthesis appears to require enzymes which act subsequent to the purF enzyme in the purine pathway.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bochner B. R., Huang H. C., Schieven G. L., Ames B. N. Positive selection for loss of tetracycline resistance. J Bacteriol. 1980 Aug;143(2):926–933. doi: 10.1128/jb.143.2.926-933.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castilho B. A., Olfson P., Casadaban M. J. Plasmid insertion mutagenesis and lac gene fusion with mini-mu bacteriophage transposons. J Bacteriol. 1984 May;158(2):488–495. doi: 10.1128/jb.158.2.488-495.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan R. K., Botstein D., Watanabe T., Ogata Y. Specialized transduction of tetracycline resistance by phage P22 in Salmonella typhimurium. II. Properties of a high-frequency-transducing lysate. Virology. 1972 Dec;50(3):883–898. doi: 10.1016/0042-6822(72)90442-4. [DOI] [PubMed] [Google Scholar]
- Chumley F. G., Menzel R., Roth J. R. Hfr formation directed by tn10. Genetics. 1979 Apr;91(4):639–655. doi: 10.1093/genetics/91.4.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downs D. M., Roth J. R. A novel P22 prophage in Salmonella typhimurium. Genetics. 1987 Nov;117(3):367–380. doi: 10.1093/genetics/117.3.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He B., Shiau A., Choi K. Y., Zalkin H., Smith J. M. Genes of the Escherichia coli pur regulon are negatively controlled by a repressor-operator interaction. J Bacteriol. 1990 Aug;172(8):4555–4562. doi: 10.1128/jb.172.8.4555-4562.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmeyer J., Neuhard J. Metabolism of exogenous purine bases and nucleosides by Salmonella typhimurium. J Bacteriol. 1971 Apr;106(1):14–24. doi: 10.1128/jb.106.1.14-24.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong J. S., Ames B. N. Localized mutagenesis of any specific small region of the bacterial chromosome. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3158–3162. doi: 10.1073/pnas.68.12.3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes K. T., Roth J. R. Conditionally transposition-defective derivative of Mu d1(Amp Lac). J Bacteriol. 1984 Jul;159(1):130–137. doi: 10.1128/jb.159.1.130-137.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes K. T., Roth J. R. Directed formation of deletions and duplications using Mud(Ap, lac). Genetics. 1985 Feb;109(2):263–282. doi: 10.1093/genetics/109.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleckner N., Roth J., Botstein D. Genetic engineering in vivo using translocatable drug-resistance elements. New methods in bacterial genetics. J Mol Biol. 1977 Oct 15;116(1):125–159. doi: 10.1016/0022-2836(77)90123-1. [DOI] [PubMed] [Google Scholar]
- Le Gal M. L., Le Gal Y., Roche J., Hedegaard J. Purine biosynthesis: enzymatic formation of ribosylamine-5-phosphate from ribose-5-phosphate and ammonia. Biochem Biophys Res Commun. 1967 Jun 23;27(6):618–624. doi: 10.1016/s0006-291x(67)80079-2. [DOI] [PubMed] [Google Scholar]
- MAGASANIK B., KARIBIAN D. Purine nucleotide cycles and their metabolic role. J Biol Chem. 1960 Sep;235:2672–2681. [PubMed] [Google Scholar]
- Makaroff C. A., Zalkin H. Regulation of Escherichia coli purF. Analysis of the control region of a pur regulon gene. J Biol Chem. 1985 Aug 25;260(18):10378–10387. [PubMed] [Google Scholar]
- Maloy S. R., Nunn W. D. Selection for loss of tetracycline resistance by Escherichia coli. J Bacteriol. 1981 Feb;145(2):1110–1111. doi: 10.1128/jb.145.2.1110-1111.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazlen A. S., Eaton N. R. Biochemical basis for the adenine requirement of ad3 mutants of Saccharomyces. Biochem Biophys Res Commun. 1967 Mar 9;26(5):590–595. doi: 10.1016/0006-291x(67)90106-4. [DOI] [PubMed] [Google Scholar]
- Meng L. M., Kilstrup M., Nygaard P. Autoregulation of PurR repressor synthesis and involvement of purR in the regulation of purB, purC, purL, purMN and guaBA expression in Escherichia coli. Eur J Biochem. 1990 Jan 26;187(2):373–379. doi: 10.1111/j.1432-1033.1990.tb15314.x. [DOI] [PubMed] [Google Scholar]
- Messenger L. J., Zalkin H. Glutamine phosphoribosylpyrophosphate amidotransferase from Escherichia coli. Purification and properties. J Biol Chem. 1979 May 10;254(9):3382–3392. [PubMed] [Google Scholar]
- NIERLICH D. P., MAGASANIK B. REGULATION OF PURINE RIBONUCLEOTIDE SYNTHESIS BY END PRODUCT INHIBITION. THE EFFECT OF ADENINE AND GUANINE RIBONUCLEOTIDES ON THE 5'-PHOSPHORIBOSYL-PYROPHOSPHATE AMIDOTRANSFERASE OF AEROBACTER AEROGENES. J Biol Chem. 1965 Jan;240:358–365. [PubMed] [Google Scholar]
- Newell P. C., Tucker R. G. Biosynthesis of the pyrimidine moiety of thiamine. A new route of pyrimidine biosynthesis involving purine intermediates. Biochem J. 1968 Jan;106(1):279–287. doi: 10.1042/bj1060279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newell P. C., Tucker R. G. Precursors of the pyrimidine moiety of thiamine. Biochem J. 1968 Jan;106(1):271–277. doi: 10.1042/bj1060271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schendel F. J., Cheng Y. S., Otvos J. D., Wehrli S., Stubbe J. Characterization and chemical properties of phosphoribosylamine, an unstable intermediate in the de novo purine biosynthetic pathway. Biochemistry. 1988 Apr 5;27(7):2614–2623. doi: 10.1021/bi00407a052. [DOI] [PubMed] [Google Scholar]
- Schmieger H. Phage P22-mutants with increased or decreased transduction abilities. Mol Gen Genet. 1972;119(1):75–88. doi: 10.1007/BF00270447. [DOI] [PubMed] [Google Scholar]
- Smith J. M., Daum H. A., 3rd Identification and nucleotide sequence of a gene encoding 5'-phosphoribosylglycinamide transformylase in Escherichia coli K12. J Biol Chem. 1987 Aug 5;262(22):10565–10569. [PubMed] [Google Scholar]
- Smith J. M., Gots J. S. purF-lac fusion and direction of purF transcription in Escherichia coli. J Bacteriol. 1980 Sep;143(3):1156–1164. doi: 10.1128/jb.143.3.1156-1164.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tso J. Y., Hermodson M. A., Zalkin H. Glutamine phosphoribosylpyrophosphate amidotransferase from cloned Escherichia coli purF. NH2-terminal amino acid sequence, identification of the glutamine site, and trace metal analysis. J Biol Chem. 1982 Apr 10;257(7):3532–3536. [PubMed] [Google Scholar]
- Tso J. Y., Zalkin H., van Cleemput M., Yanofsky C., Smith J. M. Nucleotide sequence of Escherichia coli purF and deduced amino acid sequence of glutamine phosphoribosylpyrophosphate amidotransferase. J Biol Chem. 1982 Apr 10;257(7):3525–3531. [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Way J. C., Davis M. A., Morisato D., Roberts D. E., Kleckner N. New Tn10 derivatives for transposon mutagenesis and for construction of lacZ operon fusions by transposition. Gene. 1984 Dec;32(3):369–379. doi: 10.1016/0378-1119(84)90012-x. [DOI] [PubMed] [Google Scholar]



