Abstract
λ-exonuclease participates in DNA recombination and repair. It binds a free end of double-stranded DNA and degrades one strand in the 5′ to 3′ direction. The primary sequence does not appear to be related to any other protein, but the crystal structure shows part of λ-exonuclease to be similar to the type II restriction endonucleases PvuII and EcoRV. There is also a weaker correspondence with EcoRI, BamHI, and Cfr10I. The structure comparisons not only suggest that these enzymes all share a similar catalytic mechanism and a common structural ancestor but also provide strong evidence that the toroidal structure of λ-exonuclease encircles its DNA substrate during hydrolysis.
DNA exonucleases are essential for DNA replication, recombination, and repair (1). λ-Exonuclease is a processive 5′-3′ single-stranded DNA exonuclease that requires Mg2+ as a cofactor (2–4). The protein is encoded by bacteriophage λ and functions in recombination and repair of the viral chromosome (5–10). When a double-stranded break occurs, one of the first steps taken to repair the damage is the formation of long 3′ single-stranded overhangs. These are created by the action of λ-exonuclease and then are processed by other proteins such as RecA to give a recombinant DNA molecule (11).
The crystal structure of λ-exonuclease shows the protein to be a homotrimer with a large, tapered channel that runs parallel to the threefold axis of symmetry (12). The channel appears large enough to accommodate the DNA substrate, providing a structural rationale for the processivity of the protein (12). The overall fold of λ-exonuclease is not similar to any other protein in the protein database. There is, however, a subdomain of the protein that structurally corresponds to parts of the type II restriction endonucleases PvuII and EcoRV (12).
Type II restriction endonucleases are dimeric Mg2+-dependent enzymes that recognize and cut a specific 4- to 8-bp palindromic DNA sequence (13, 14). In conjunction with a methylase that recognizes the same DNA sequence, these two proteins make up a bacterial Restriction Modification System that is thought to protect the bacterium from foreign DNA, such as invading viral DNA. To date, there are crystal structures of five type II restriction endonucleases: PvuII and EcoRV, which cleave DNA to give blunt ends, and EcoRI, BamHI, and Cfr10I, which cut DNA to give recessed ends (15–20). There is very little homology at the primary sequence level between restriction endonucleases. However, as pointed out by Venclovas et al. (21), at the structural level these five enzymes share a common motif consisting of five β-strands sandwiched between two α-helices. The blunt-end cutters EcoRV and PvuII are more similar to each other than to the recessed-end cutters EcoRI, BamHI, and Cfr10I (15, 20).
Structural Similarity Between λ-Exonuclease and the Type II Restriction Enzymes.
The structural relationship between λ-exonuclease (Brookhaven Data Bank ID Code 1AVQ) and the type II restriction endonucleases EcoRV and PvuII was identified by using the Dali structural database server (22) and is illustrated in Fig. 1. There are 78 equivalent Cα positions in PvuII and λ-exonuclease with a rms deviation of 2.7 Å. Twelve of the structurally equivalent residues are chemically identical (Table 1). Similarly, Dali identified 90 equivalent Cα positions in EcoRV and λ-exonuclease with an rms deviation of 4.5 Å. Ten of the 94 residues are identical.
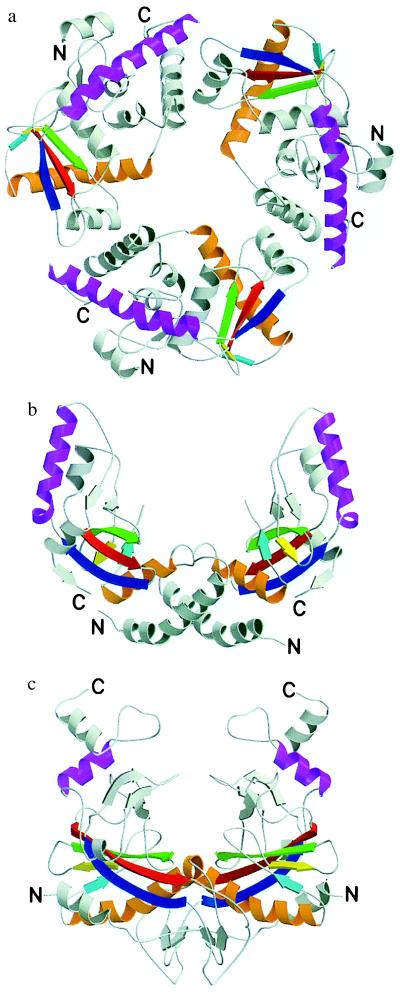
Figure 1.
Ribbon diagrams of λ-exonuclease (a), PvuII (b), and EcoRV (c) with corresponding secondary structure elements between the three proteins colored similarly. Figure created with molscript (26) and rendered in raster3d (27, 28).
Table 1.
Structural relationships between λ-exonuclease and type II restriction enzymes
| PvuII (15, 16) | EcoRV (17) | EcoRI (18) | BamHI (19) | Cfr10I (20) | |
|---|---|---|---|---|---|
| Structurally equivalent residues | 78 | 90 | 51 | 63 | 74 |
| Deviation between Cα atoms, Å | 2.7 | 4.5 | 3.5 | 4.1 | 3.0 |
| Chemically identical residues | 12 | 10 | 2 | 8 | 6 |
| Corresponding active-site residues | |||||
| λ-exonuclease: Glu-85 | Glu-55 | Glu-45 | Asp-59 | Glu-77 | Glu-71 |
| λ-exonuclease: Asp-119 | Asp-58 | Asp-74 | Asp-91 | Asp-94 | Asp-134 |
| λ-exonuclease: Glu-129 | Glu-68 | Asp-90 | Clu-111 | Glu-111 | Glu-204 |
| λ-exonuclease: Lys-131 | Lys-70 | Lys-92 | Lys-113 | Glu-11 | Lys-190 |
| Structurally equivalent residues | |||||
| λ-exonuclease: αE | 83–98, 33–48 | 74–97, 34–57 | 79–97, 33–51 | 80–97, 56–73 | 74–96, 60–82 |
| λ-exonuclease: βa | 99–103, 49–53 | 98–106, 61–69 | 98–109, 53–64 | 100–103, 74–77 | 98–103, 89–94 |
| λ-exonuclease: βc | — | — | 111–117, 83–89 | — | 113–118, 128–133 |
| λ-exonuclease: βd | 121–124, 58–61 | 116–124, 71–79 | 119–124, 94–99 | 117–124, 92–99 | 119–123, 134–138 |
| λ-exonuclease: βe | 125–135, 66–76 | 125–132, 86–93 | 125–131, 104–110 | 125–133, 107–115 | 124–133, 183–192 |
| λ-exonuclease: loop | — | 134–137, 94–97 | — | — | — |
| λ-exonuclease: αG | — | — | — | 149–165, 118–134 | 148–166, 195–213 |
| λ-exonuclease: loop | 163–166, 91–94 | 165–168, 125–128 | — | 166–169, 135–138 | — |
| λ-exonuclease: βf | 167–177, 96–106 | 169–176, 130–137 | — | 170–174, 139–143 | 168–175, 227–234 |
| λ-exonuclease: βg | 183–191, 109–117 | 183–189, 167–173 | — | — | — |
| λ-exonuclease: αH | 192–207, 120–135 | 192–202, 209–219 | — | — | — |
| λ-exonuclease: αH | — | 205–210, 220–225 | — | — | — |
Each of the proteins listed was compared with λ-exonuclease using the Dali database server (22). The table gives the number of equivalent residues, the rms deviation, and the number of structurally equivalent residues that are chemically identical in the pairwise comparison of λ-exonuclease with each enzyme. The segments of structure that correspond are identified, with the residues from λ-exonuclease given first, followed by the structurally equivalent residues in the restriction enzyme. In addition to the overall regions of similarity, the server identified residues 154–157 in λ-exonuclease and residues 80–83 in PvuII and residues 153–156 in λ-exonuclease and residues 106–109 in EcoRV as equivalent. However, by visual inspection, it was clear that these short segments are not part of the overall correspondence, and they therefore were deleted from the analysis. Similar short segments were identified by Dali in the pairwise comparison of λ-exonuclease with EcoRI, BamHI, and Cfr10I and also were omitted.
The region of homology among λ exonuclease, EcoRV, and PvuII consists of five β-strands and two α-helices (Figs. 1–3). The region of correspondence begins with helix αE and strands βa, βd, and βe in λ-exonuclease. These are homologous to helix αB and strands βa, βb, and βc in PvuII and to helix αB and strands βc, βd, and βe in EcoRV. There is then an interruption in the structural correspondence between λ-exonuclease and both EcoRV and PvuII. The similarity begins again with strands of βf, βg, and helix αH. These regions are similar to βe, βf, and αD in PvuII and βg, βh, and αD in EcoRV.
Figure 3.
Stereoview overlay of Cα atoms for λ-exonuclease (yellow) and PvuII (cyan) protein fragments (a) and λ-exonuclease (yellow) and EcoRV (magenta) protein fragments (b). The segments shown include all of the regions of structural similarity identified by the Dali database server (Table 1). Figure prepared by using molscript (26) and rendered with raster3d (27, 28).
Helix αB in PvuII and EcoRV (shaded orange in Fig. 1) forms parts of the dimer interface of these two proteins. However, the homologous helix in λ-exonuclease, αE, is located on the “back” of the protein (Fig. 1) and does not participate in any monomer–monomer contacts. The interface between monomers in λ-exonuclease is formed by helix αF and a loop region (residues 175–184) between strands βf and βg. Because of its homology to helix αB in PvuII and EcoRV and its relatively exposed location, we speculate that helix αE in λ-exonuclease may be involved in other protein–protein interactions. In particular, in vitro data suggest that λ-exonuclease forms a complex with another bacteriophage λ-encoded protein termed “β” that also is involved in phage recombination (4). We suggest that the interface formed between the two proteins might include helix αE of λ-exonuclease.
It previously has been noted that, notwithstanding the similarity in the region of their active sites, the dimerization domains of PvuII and EcoRV differ substantially (16). As shown in Fig. 1, the present comparisons expand and extend this pattern of behavior. Not only are different regions of λ-exonuclease used to make the subunit–subunit interfaces but the character of these interfaces changes from “face-to-face” dimerization in the restriction enzyme to “head-to-tail” association in the λ-exonuclease trimer.
Relationships with EcoRI, BamHI, and Cfr10I.
λ-exonuclease is a more distant structural relative to the recessed-end cutters EcoRI, BamHI, and Cfr10I than it is to EcoRV and PvuII. These type II restriction endonucleases were not identified as structural homologues by the Dali database search. They do, however, share the motif of five β-strands flanked by two α-helices that is common to λ-exonuclease, PvuII, and EcoRV. The Dali server (22) was used again to compare λ-exonuclease with EcoRI, BamHI, and Cfr10I (Table 1). In distinction to all the other comparisons, Dali identified helix αG of λ-exonuclease as corresponding to helix α7 of Cfr10I and helix α4 of BamHI. The correspondence between αG in λ-exonuclease and α4 in BamHI, however, is much less compelling than that of αG in λ-exonuclease with α7 in Cfr10I. Bozic et al. (20) have speculated that the helix α7 in Cfr10I may be involved in interactions with the DNA. This argument is based in part on the structural correspondence to helix α4 of EcoRI. Helix α4 of EcoRI does not, however, correspond structurally with helix αG of λ-exonuclease. Whether this helix in λ-exonuclease is important for protein–DNA interaction is not known.
Correspondence of Active Site Residues.
Based on the above structural similarity, the residues Asp-119, Glu-129, and Lys-131 in the active site of λ-exonuclease can be identified as corresponding, respectively, to Asp-74, Asp-90, and Lys-92 in EcoRV and to Asp-58, Glu-68, and Lys-70 in PvuII. There are also analogous active-site residues for EcoRI, BamHI, and Cfr10I (Table 1). By using the program edpdb (23), we made a comparison by overlaying all of the atoms of residues Asp-119, Glu-129, and Lys-131 in λ-exonuclease with all of the atoms in residues Asp-58, Glu-68, and Lys-70 in PvuII. A related comparison was made with Asp-74, Asp-90, and Lys-92 in EcoRV (Fig. 4A). To compare Glu-129 in λ-exonuclease with Asp-90 in EcoRV, the Cγ atom of Glu-129 was excluded. The rms discrepancy for the 26 atoms involved was 0.95 Å for PvuII and 1.17 Å for EcoRV.
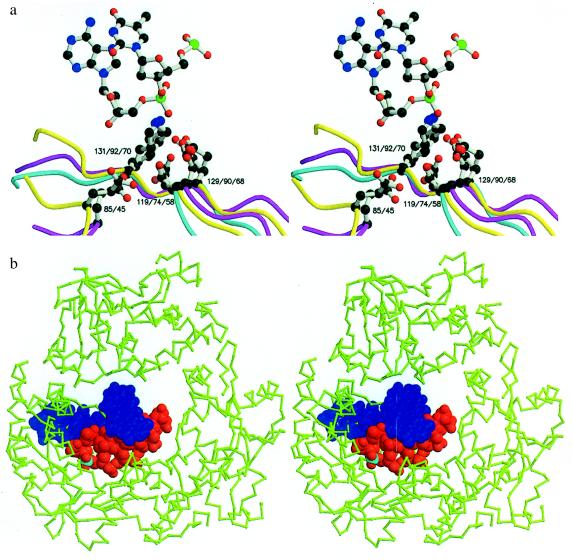
Figure 4.
(A) Stereoview showing the superposition of the active site residues of λ-exonuclease (yellow), (Glu-85, Asp-119, Glu-129, and Lys-131), PvuII (cyan) (Asp-58, Glu-68, and Lys-70), and EcoRV (magenta) (Glu-45, Asp-74, Asp-90, and Lys-92). The figure includes the central nucleotide (TA) from the co-crystal structure of EcoRV bound to its cognate DNA sequence (17). (B) Superposition on the trimeric structure of λ-exonuclease of the cognate DNA decamer from the EcoRV:DNA complex (17). To make the DNA resemble the substrate of λ-exonucleases, five nucleotides were removed from the 5′ end of the strand that is hydrolyzed. The next phosphate that would be attacked is shown in cyan. The DNA was aligned based on the correspondence between the active site residues of EcoRV and λ-exonuclease (A). In the stereoview, the wider end of the central channel through the λ-exonuclease trimer, i.e., the end of the channel that is thought to accept the double-strand DNA substrate, is toward the viewer. Figure created by using molscript (26) and rendered with raster3d (27, 28).
It has been shown for all six proteins that two of the acidic active site residues form a binding site for the catalytically essential magnesium ion (12, 15–20). In the co-crystal structure of EcoRV bound to its specific DNA sequence, under some conditions, a second metal binding site is formed by Asp-90 and Glu-45 (24). In λ-exonuclease, there is a glutamic acid (Glu-85) that structurally corresponds to Glu-45 of EcoRV. Whether a second magnesium binding site is formed and, if so, whether it participates in catalysis remain uncertain. In general terms, however, the correspondence between the active sites strongly suggests that the mechanism of catalysis of λ-exonuclease is very similar to those of PvuII and EcoRV.
Implications for the Mechanism of Action of λ-Exonuclease.
Several aspects of the present work have specific implications for the mechanism of action of λ-exonuclease. First, the structure comparisons confirm the presumed location of the active site (12) and show it to be on the inside of the toroidal trimer. Second, the comparisons are consistent with the presumed alignment of the hydrolyzed DNA strand. Fig. 4A includes the central dinucleotide (TA) from the EcoRV:DNA complex aligned within the active site of λ-exonuclease, based on the structural similarity of the two proteins. The location and mode of binding are as expected for cleavage by λ-exonuclease in the 5′ to 3′ direction. Extending this result, we took the entire DNA decamer as seen in complex with EcoRV (17), trimmed part of one strand so as to correspond to the substrate for λ-exonuclease, and used the structural alignment between EcoRV and λ-exonuclease to place this segment of DNA on the structure of λ-exonuclease. In this superposition (Fig. 4B), the double-strand part of the DNA is located within the wider end of the channel that passes through the λ-exonuclease toroid and the single-strand end of the DNA is located within the narrow end of the channel. This alignment of the DNA, determined by superposition of corresponding structural elements of λ-exonuclease and EcoRV, is very similar to that suggested by consideration of the λ-exonuclease structure alone (12). It provides independent support for the idea that the λ-exonuclease toroid encircles its DNA substrate during hydrolysis.
The structural homology between λ-exonuclease and the type II restriction endonucleases PvuII, EcoRV, EcoRI, BamHI, and Cfr10I also suggests that each of these proteins diverged from a common structural ancestor. Recently, Ban and Yang (25) showed that the similarity between PvuII and EcoRV extends to include the restriction enzyme FokI and the monomeric sequence-specific endonuclease MutH. It is ironic that the type II restriction endonucleases, which protect bacteria by hydrolyzing invading viral DNA, and an exonuclease that is involved in recombination and repair of the same phage DNA, both appear to have evolved from a common structural ancestor.
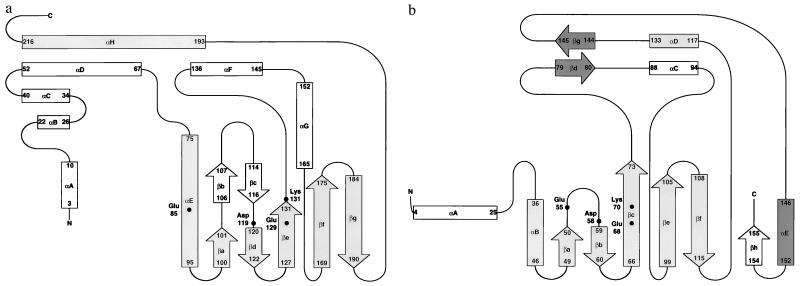
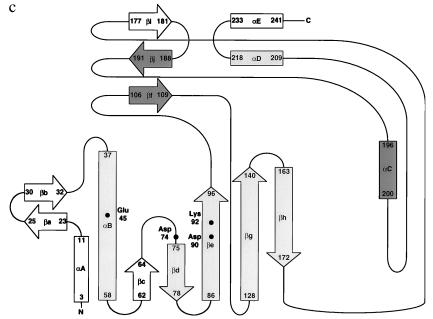
Figure 2.
Schematic representations of the topology of the secondary structure for λ-exonuclease (a), PvuII (b), and EcoRV (c). Regions that structurally correspond in all three proteins are shaded in gray. Regions of structural similarity between PvuII and EcoRV that are not seen in λ-exonuclease are shaded in dark gray. b and c are modified from refs. 15–17. Regions of secondary structure for λ-exonuclease were determined by using the program dssp (29). Structurally corresponding active site residues also are identified.
Acknowledgments
We thank Dr. Michael Quillin for comments on the manuscript and Doug Juers for advice on the structural comparisons. This work was supported in part by National Institutes of Health Grant GM20066 to B.W.M.
References
- 1.Linn S M, Lloyd R S, Roberts R J, editors. Nucleases. Plainview, NY: Cold Spring Harbor Lab. Press; 1993. [Google Scholar]
- 2.Radding C M. J Mol Biol. 1966;18:235–250. doi: 10.1016/s0022-2836(66)80243-7. [DOI] [PubMed] [Google Scholar]
- 3.Little J W. J Biol Chem. 1967;242:679–686. [PubMed] [Google Scholar]
- 4.Carter D M, Radding C M. J Biol Chem. 1971;246:2502–2512. [PubMed] [Google Scholar]
- 5.Cassuto E, Radding C M. Nat N Biol. 1971;229:13–16. doi: 10.1038/newbio229013a0. [DOI] [PubMed] [Google Scholar]
- 6.Cassuto E, Lash T, Sriprakash K S, Radding C M. Proc Natl Acad Sci USA. 1971;68:1639–1643. doi: 10.1073/pnas.68.7.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stahl F W, Kobayashi I, Stahl M M. J Mol Biol. 1985;181:199–209. doi: 10.1016/0022-2836(85)90085-3. [DOI] [PubMed] [Google Scholar]
- 8.Stahl F W, Stahl M M. J Genet. 1985;64:31–39. [Google Scholar]
- 9.Thaler D S, Stahl M M, Stahl F W. J Mol Biol. 1987;195:75–87. doi: 10.1016/0022-2836(87)90328-7. [DOI] [PubMed] [Google Scholar]
- 10.Takahashi N, Kobayashi I. Proc Natl Acad Sci USA. 1990;87:2790–2794. doi: 10.1073/pnas.87.7.2790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kowalczykowski S C, Dixon D A, Eggleston A K, Lauder S D, Rehrauer W M. Microbiol Rev. 1994;58:401–465. doi: 10.1128/mr.58.3.401-465.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kovall R, Matthews B W. Science. 1997;277:1824–1827. doi: 10.1126/science.277.5333.1824. [DOI] [PubMed] [Google Scholar]
- 13.Aggarwal A K. Curr Opin Struct Biol. 1995;5:11–19. doi: 10.1016/0959-440x(95)80004-k. [DOI] [PubMed] [Google Scholar]
- 14.Pingoud A, Jeltsch A. Eur J Biochem. 1997;246:1–22. doi: 10.1111/j.1432-1033.1997.t01-6-00001.x. [DOI] [PubMed] [Google Scholar]
- 15.Athanasiadis A, Vlassi M, Kotsifaki D, Tucker P A, Wilson K S, Kokkinidis M. Nat Struct Biol. 1994;1:469–475. doi: 10.1038/nsb0794-469. [DOI] [PubMed] [Google Scholar]
- 16.Cheng X, Balendiran K, Schildkraut I, Anderson J E. EMBO J. 1994;13:3927–3935. doi: 10.1002/j.1460-2075.1994.tb06708.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Winkler F K, Banner D W, Oefner C, Tsernoglou D, Brown R S, Heathman S P, Bryan R K, Martin P D, Petratos K, Wilson K S. EMBO J. 1993;12:1781–1795. doi: 10.2210/pdb4rve/pdb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim Y, Grable J C, Love R, Greene P J, Rosenberg J M. Science. 1990;249:1307–1309. doi: 10.1126/science.2399465. [DOI] [PubMed] [Google Scholar]
- 19.Newman M, Strzelecka T, Dorner L F, Schildkraut I, Aggarwal A K. Nature (London) 1994;368:660–664. doi: 10.1038/368660a0. [DOI] [PubMed] [Google Scholar]
- 20.Bozic D, Grazulis S, Siksnys V, Huber R. J Mol Biol. 1996;255:176–186. doi: 10.1006/jmbi.1996.0015. [DOI] [PubMed] [Google Scholar]
- 21.Venclovas C, Timinskas A, Siksnys V. Proteins. 1994;20:279–282. doi: 10.1002/prot.340200308. [DOI] [PubMed] [Google Scholar]
- 22.Holm L, Sander C. J Mol Biol. 1993;233:123–138. doi: 10.1006/jmbi.1993.1489. [DOI] [PubMed] [Google Scholar]
- 23.Zhang X-J, Matthews B W. J Appl Cryst. 1995;28:624–630. [Google Scholar]
- 24.Kostrewa D, Winkler F K. Biochemistry. 1995;34:683–696. doi: 10.1021/bi00002a036. [DOI] [PubMed] [Google Scholar]
- 25.Ban C, Yang W. EMBO J. 1998;17:1526–1534. doi: 10.1093/emboj/17.5.1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kraulis P J. J Appl Cryst. 1991;24:946–950. [Google Scholar]
- 27.Bacon D J, Anderson W F. Mol Graphics. 1988;6:219–220. [Google Scholar]
- 28.Merritt E A, Murphy M E P. Acta Cryst. 1994;D 50:869–873. doi: 10.1107/S0907444994006396. [DOI] [PubMed] [Google Scholar]
- 29.Kabsch W, Sander C. Biopolymers. 1983;22:2577–2637. doi: 10.1002/bip.360221211. [DOI] [PubMed] [Google Scholar]