Abstract
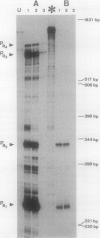
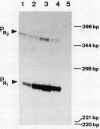
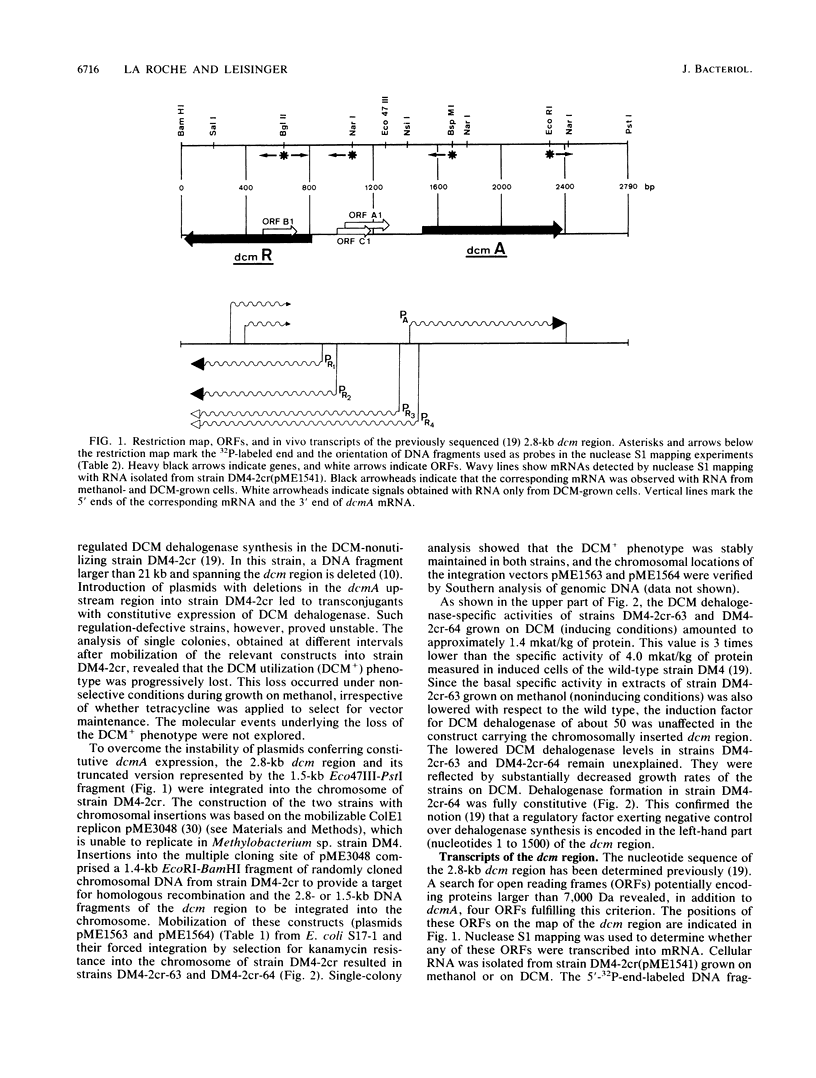
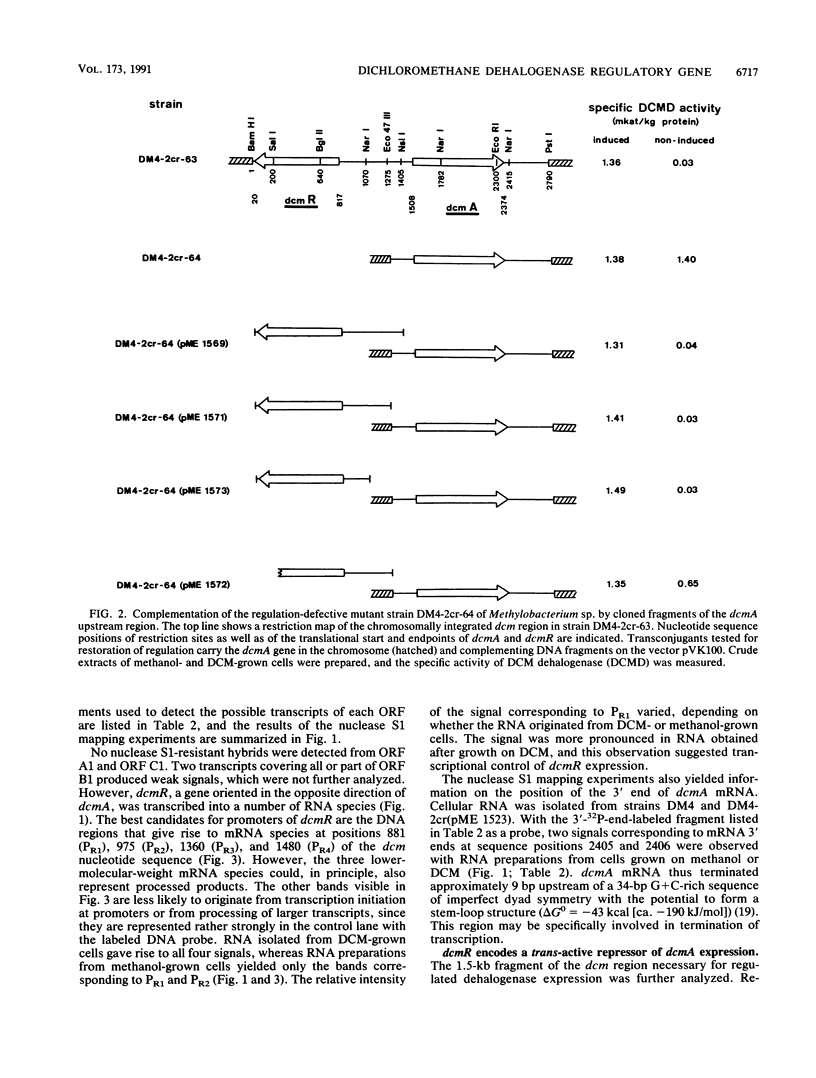
The genes for dichloromethane utilization by Methylobacterium sp. strain DM4 are encoded on a 2.8-kb sequenced DNA fragment, the dcm region. This fragment contains dcmA, the structural gene of dichloromethane dehalogenase and, upstream of dcmA, a 1.5-kb region responsible for inducibility of dichloromethane dehalogenase by dichloromethane. A fragment of the dcm region covering dcmA and 230 bp of its upstream region was integrated into the chromosome of a Methylobacterium sp. strain DM4 mutant deleted for the dcm region. This yielded a strain expressin dichloromethane dehalogenase constitutively at the induced level. Plasmids carrying various segments of the 1.5-kb regulatory region were tested for their ability to restore regulation. The data obtained led to the identification of dcmR, the structural gene of a putative dcm-specific repressor. Transcription of dcmR was divergent from dcmA. dcmR encoded a 30-kDa protein with a helix-turn-helix motif near the amino terminus. The transcription start sites of dcmA and dcmR were identified by nuclease S1 mapping. The promoter regions of these genes contained nearly identical 12-bp sequences covering positions -14 to -25 relative to the mRNA start sites. Experiments with dcmR'-'lacZ fusions demonstrated that dcmR expression was markedly autoregulated at the level of transcription and less so at the protein level. These findings are compatible with both dcmA and dcmR expression being negatively controlled at the transcriptional level by the DcmR protein.
Full text
PDF

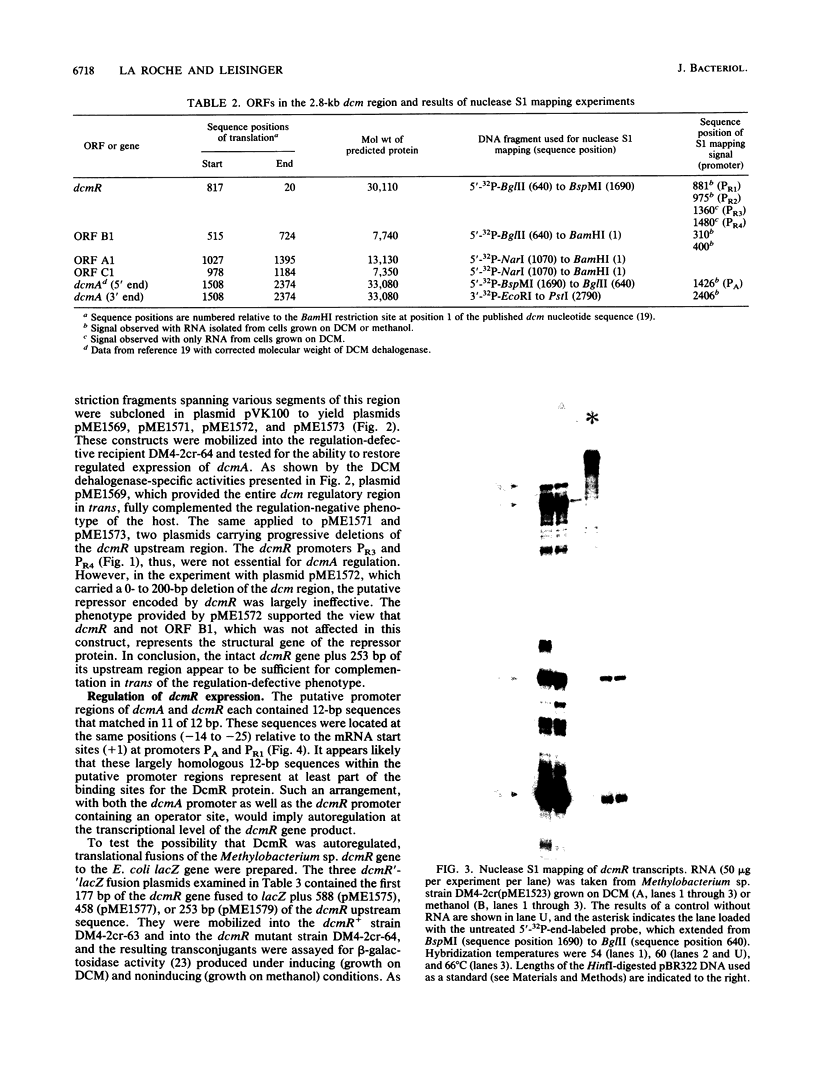
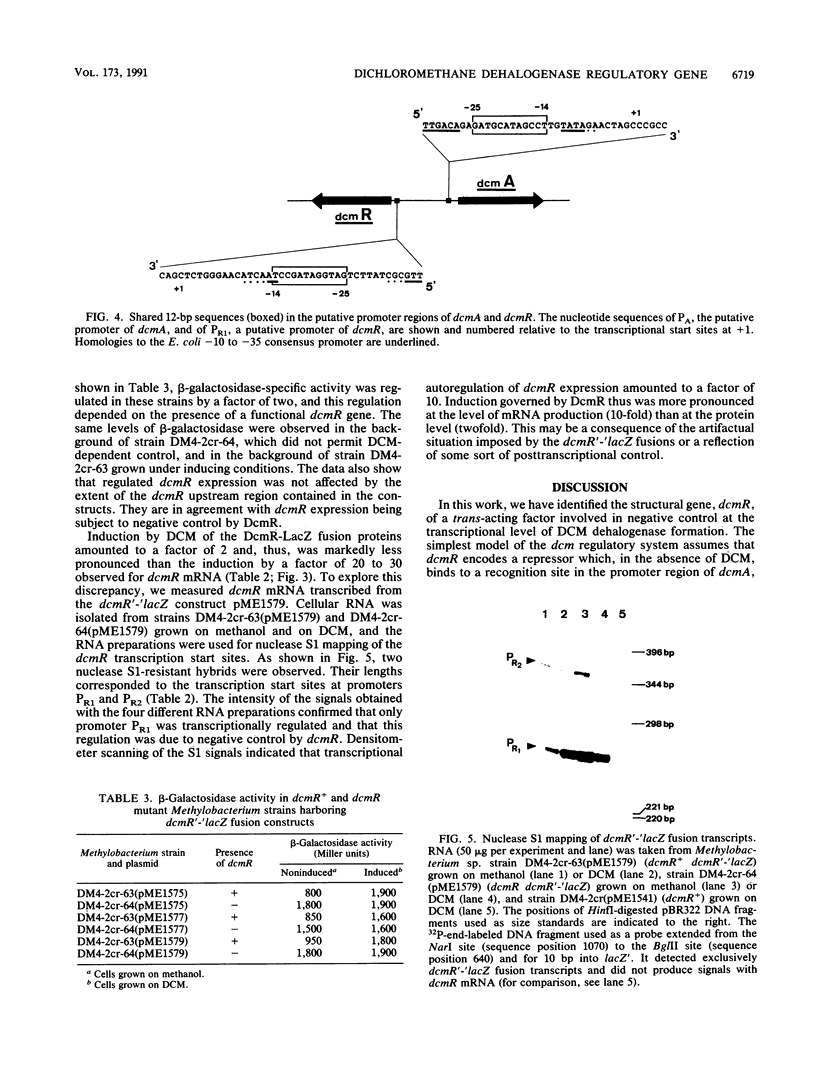


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adhya S. Multipartite genetic control elements: communication by DNA loop. Annu Rev Genet. 1989;23:227–250. doi: 10.1146/annurev.ge.23.120189.001303. [DOI] [PubMed] [Google Scholar]
- Allison S. L., Phillips A. T. Nucleotide sequence of the gene encoding the repressor for the histidine utilization genes of Pseudomonas putida. J Bacteriol. 1990 Sep;172(9):5470–5476. doi: 10.1128/jb.172.9.5470-5476.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assinder S. J., Williams P. A. The TOL plasmids: determinants of the catabolism of toluene and the xylenes. Adv Microb Physiol. 1990;31:1–69. doi: 10.1016/s0065-2911(08)60119-8. [DOI] [PubMed] [Google Scholar]
- Beck C. F., Warren R. A. Divergent promoters, a common form of gene organization. Microbiol Rev. 1988 Sep;52(3):318–326. doi: 10.1128/mr.52.3.318-326.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadaban M. J., Martinez-Arias A., Shapira S. K., Chou J. Beta-galactosidase gene fusions for analyzing gene expression in escherichia coli and yeast. Methods Enzymol. 1983;100:293–308. doi: 10.1016/0076-6879(83)00063-4. [DOI] [PubMed] [Google Scholar]
- Dodd I. B., Egan J. B. Improved detection of helix-turn-helix DNA-binding motifs in protein sequences. Nucleic Acids Res. 1990 Sep 11;18(17):5019–5026. doi: 10.1093/nar/18.17.5019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd I. B., Egan J. B. Systematic method for the detection of potential lambda Cro-like DNA-binding regions in proteins. J Mol Biol. 1987 Apr 5;194(3):557–564. doi: 10.1016/0022-2836(87)90681-4. [DOI] [PubMed] [Google Scholar]
- Eggink G., Engel H., Meijer W. G., Otten J., Kingma J., Witholt B. Alkane utilization in Pseudomonas oleovorans. Structure and function of the regulatory locus alkR. J Biol Chem. 1988 Sep 15;263(26):13400–13405. [PubMed] [Google Scholar]
- Gälli R., Leisinger T. Plasmid analysis and cloning of the dichloromethane-utilization genes of Methylobacterium sp. DM4. J Gen Microbiol. 1988 Apr;134(4):943–952. doi: 10.1099/00221287-134-4-943. [DOI] [PubMed] [Google Scholar]
- Henikoff S., Haughn G. W., Calvo J. M., Wallace J. C. A large family of bacterial activator proteins. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6602–6606. doi: 10.1073/pnas.85.18.6602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennecke H., Günther I., Binder F. A novel cloning vector for the direct selection of recombinant DNA in E. coli. Gene. 1982 Sep;19(2):231–234. doi: 10.1016/0378-1119(82)90011-7. [DOI] [PubMed] [Google Scholar]
- Hood D. W., Dow C. S., Green P. N. DNA:DNA hybridization studies on the pink-pigmented facultative methylotrophs. J Gen Microbiol. 1987 Mar;133(3):709–720. doi: 10.1099/00221287-133-3-709. [DOI] [PubMed] [Google Scholar]
- Janssen D. B., Pries F., van der Ploeg J., Kazemier B., Terpstra P., Witholt B. Cloning of 1,2-dichloroethane degradation genes of Xanthobacter autotrophicus GJ10 and expression and sequencing of the dhlA gene. J Bacteriol. 1989 Dec;171(12):6791–6799. doi: 10.1128/jb.171.12.6791-6799.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen R. A., Rothstein S. J., Reznikoff W. S. A restriction enzyme cleavage map of Tn5 and location of a region encoding neomycin resistance. Mol Gen Genet. 1979;177(1):65–72. doi: 10.1007/BF00267254. [DOI] [PubMed] [Google Scholar]
- Kelley R. L., Yanofsky C. Trp aporepressor production is controlled by autogenous regulation and inefficient translation. Proc Natl Acad Sci U S A. 1982 May;79(10):3120–3124. doi: 10.1073/pnas.79.10.3120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knauf V. C., Nester E. W. Wide host range cloning vectors: a cosmid clone bank of an Agrobacterium Ti plasmid. Plasmid. 1982 Jul;8(1):45–54. doi: 10.1016/0147-619x(82)90040-3. [DOI] [PubMed] [Google Scholar]
- La Roche S. D., Leisinger T. Sequence analysis and expression of the bacterial dichloromethane dehalogenase structural gene, a member of the glutathione S-transferase supergene family. J Bacteriol. 1990 Jan;172(1):164–171. doi: 10.1128/jb.172.1.164-171.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy J. E., Gualerzi C. Translational control of prokaryotic gene expression. Trends Genet. 1990 Mar;6(3):78–85. doi: 10.1016/0168-9525(90)90098-q. [DOI] [PubMed] [Google Scholar]
- Minton N. P. Improved plasmid vectors for the isolation of translational lac gene fusions. Gene. 1984 Nov;31(1-3):269–273. doi: 10.1016/0378-1119(84)90220-8. [DOI] [PubMed] [Google Scholar]
- Schell M. A., Poser E. F. Demonstration, characterization, and mutational analysis of NahR protein binding to nah and sal promoters. J Bacteriol. 1989 Feb;171(2):837–846. doi: 10.1128/jb.171.2.837-846.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholtz R., Wackett L. P., Egli C., Cook A. M., Leisinger T. Dichloromethane dehalogenase with improved catalytic activity isolated from a fast-growing dichloromethane-utilizing bacterium. J Bacteriol. 1988 Dec;170(12):5698–5704. doi: 10.1128/jb.170.12.5698-5704.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szeto W. W., Nixon B. T., Ronson C. W., Ausubel F. M. Identification and characterization of the Rhizobium meliloti ntrC gene: R. meliloti has separate regulatory pathways for activation of nitrogen fixation genes in free-living and symbiotic cells. J Bacteriol. 1987 Apr;169(4):1423–1432. doi: 10.1128/jb.169.4.1423-1432.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]