Abstract
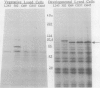
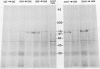
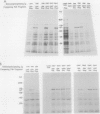
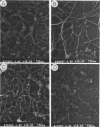
We have been using monoclonal antibodies (MAbs) as probes to study developmentally relevant cell surface antigens (CSA) that may be required for cellular interactions in Myxococcus xanthus. Three independently isolated MAbs, G69, G357, and G645, isolated by Gill and Dworkin recognize a CSA detectable only on developing cells (J. S. Gill and M. Dworkin, J. Bacteriol. 168:505-511, 1986). The CSA is made within the first 30 min of submerged development and increases until myxosporulation. The CSA is also produced at low levels after 24 h in shaken-starved cultures and during glycerol sporulation. No antigen can be detected in lysed, vegetative cells, and expression of the antigen is blocked in the presence of rifampin or chloramphenicol. The antigen is expressed in submerged, developmental cultures of asg, bsg, csg, dsg, and mgl mutants and is not expressed in a dsp mutant. All of the three MAbs immunoprecipitate the same protein of approximately 97,000 Da from lysed developmental cells. Competitive immunoprecipitations suggest that they recognize at least two different epitopes on the CSA. The epitopes recognized by MAbs G69, G357, and G645 are sensitive to protease digestion, whereas the epitopes recognized by MAbs G357 and G645 are resistant to periodate oxidation. The epitope recognized by MAb G69 is sensitive to periodate oxidation. Fractionation of lysed developing cells shows that most of the antigen is localized in the pellet after centrifugation at 100,000 x g. To determine whether the antigen is expressed on the cell surface, we labeled developing whole cells with either MAb G69, G357, or G645 and gold-labeled anti-mouse immunoglobulin G. Low-voltage scanning electron microscopy of labeled cells shows that the antigen is associated with the fibrillar matrix that surrounds the cells and that the antigen is retained on isolated, developmental fibrils from M. xanthus. The CSA has been designated dFA-1, for developmental fibrillar antigen 1.
Full text
PDF





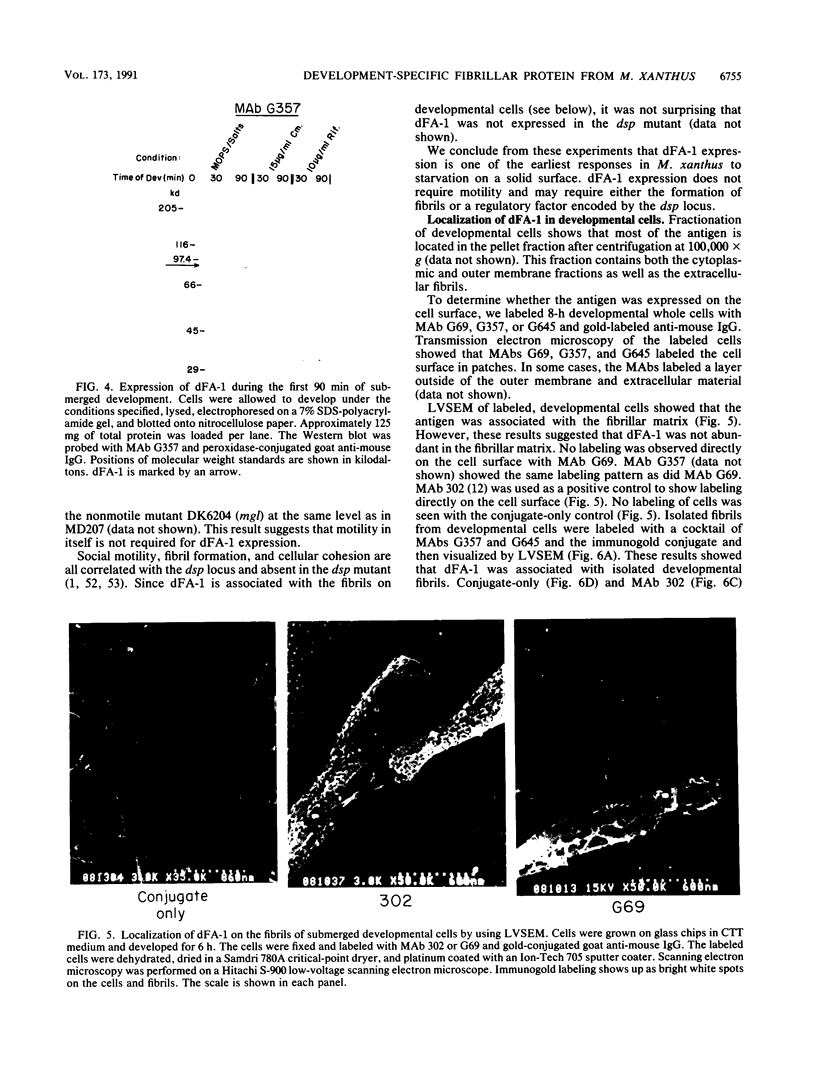
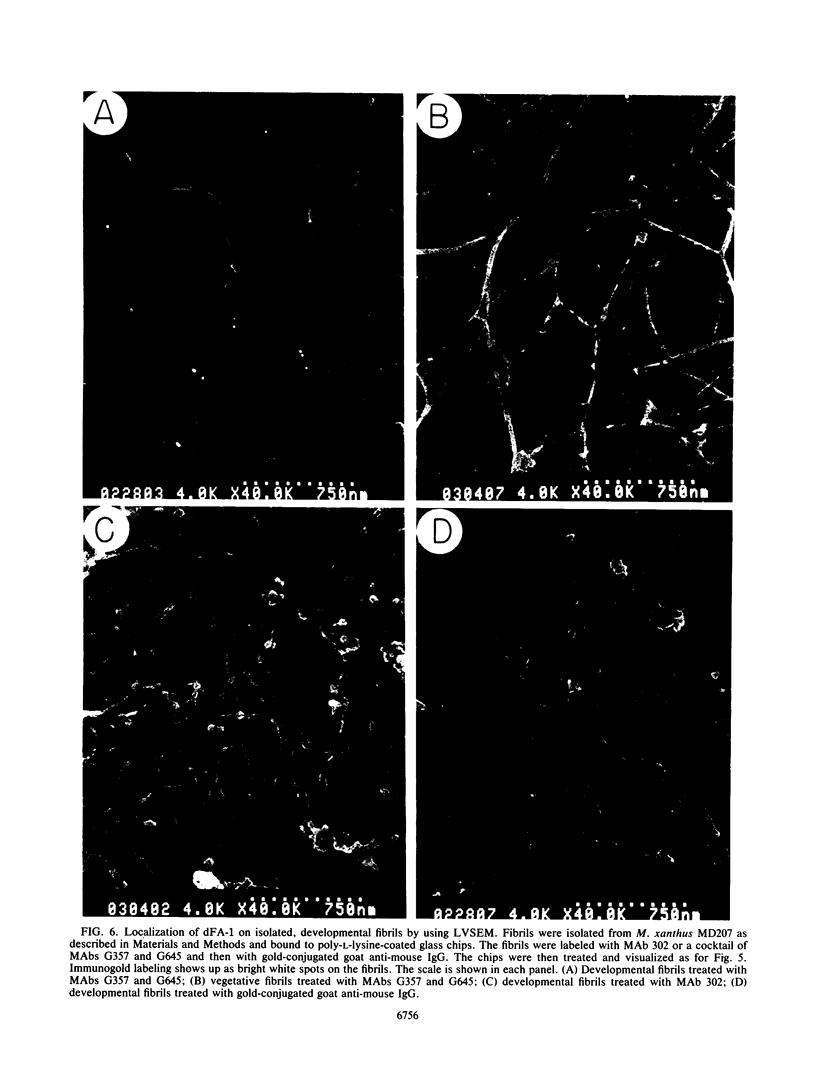



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnold J. W., Shimkets L. J. Cell surface properties correlated with cohesion in Myxococcus xanthus. J Bacteriol. 1988 Dec;170(12):5771–5777. doi: 10.1128/jb.170.12.5771-5777.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold J. W., Shimkets L. J. Inhibition of cell-cell interactions in Myxococcus xanthus by congo red. J Bacteriol. 1988 Dec;170(12):5765–5770. doi: 10.1128/jb.170.12.5765-5770.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bieber A. J., Snow P. M., Hortsch M., Patel N. H., Jacobs J. R., Traquina Z. R., Schilling J., Goodman C. S. Drosophila neuroglian: a member of the immunoglobulin superfamily with extensive homology to the vertebrate neural adhesion molecule L1. Cell. 1989 Nov 3;59(3):447–460. doi: 10.1016/0092-8674(89)90029-9. [DOI] [PubMed] [Google Scholar]
- Cheng Y., Kaiser D. dsg, a gene required for cell-cell interaction early in Myxococcus development. J Bacteriol. 1989 Jul;171(7):3719–3726. doi: 10.1128/jb.171.7.3719-3726.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DWORKIN M., GIBSON S. M. A SYSTEM FOR STUDYING MICROBIAL MORPHOGENESIS: RAPID FORMATION OF MICROCYSTS IN MYXOCOCCUS XANTHUS. Science. 1964 Oct 9;146(3641):243–244. doi: 10.1126/science.146.3641.243. [DOI] [PubMed] [Google Scholar]
- DWORKIN M. Nutritional requirements for vegetative growth of Myxococcus xanthus. J Bacteriol. 1962 Aug;84:250–257. doi: 10.1128/jb.84.2.250-257.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobson W. J., McCurdy H. D. The function of fimbriae in Myxococcus xanthus. I. Purification and properties of M. xanthus fimbriae. Can J Microbiol. 1979 Oct;25(10):1152–1160. doi: 10.1139/m79-179. [DOI] [PubMed] [Google Scholar]
- Fink J. M., Kalos M., Zissler J. F. Isolation of cell surface antigen mutants of Myxococcus xanthus by use of monoclonal antibodies. J Bacteriol. 1989 Apr;171(4):2033–2041. doi: 10.1128/jb.171.4.2033-2041.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink J. M., Zissler J. F. Characterization of lipopolysaccharide from Myxococcus xanthus by use of monoclonal antibodies. J Bacteriol. 1989 Apr;171(4):2028–2032. doi: 10.1128/jb.171.4.2028-2032.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill J. S., Dworkin M. Cell surface antigens during submerged development of Myxococcus xanthus examined with monoclonal antibodies. J Bacteriol. 1986 Nov;168(2):505–511. doi: 10.1128/jb.168.2.505-511.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill J. S., Dworkin M. Isolation of additional monoclonal antibodies directed against cell surface antigens of Myxococcus xanthus cells undergoing submerged development. J Bacteriol. 1988 Dec;170(12):5953–5955. doi: 10.1128/jb.170.12.5953-5955.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill J. S., Jarvis B. W., Dworkin M. Inhibition of development in Myxococcus xanthus by monoclonal antibody 1604. Proc Natl Acad Sci U S A. 1987 Jul;84(13):4505–4508. doi: 10.1073/pnas.84.13.4505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill J., Stellwag E., Dworkin M. Monoclonal antibodies against cell-surface antigens of developing cells of Myxococcus xanthus. Ann Inst Pasteur Microbiol. 1985 Jan-Feb;136A(1):11–18. doi: 10.1016/s0769-2609(85)80015-6. [DOI] [PubMed] [Google Scholar]
- Hanson M. S., Brinton C. C., Jr Identification and characterization of E. coli type-1 pilus tip adhesion protein. Nature. 1988 Mar 17;332(6161):265–268. doi: 10.1038/332265a0. [DOI] [PubMed] [Google Scholar]
- Hodgkin J., Kaiser D. Cell-to-cell stimulation of movement in nonmotile mutants of Myxococcus. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2938–2942. doi: 10.1073/pnas.74.7.2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inouye M., Inouye S., Zusman D. R. Biosynthesis and self-assembly of protein S, a development-specific protein of Myxococcus xanthus. Proc Natl Acad Sci U S A. 1979 Jan;76(1):209–213. doi: 10.1073/pnas.76.1.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inouye M., Inouye S., Zusman D. R. Gene expression during development of Myxococcus xanthus: pattern of protein synthesis. Dev Biol. 1979 Feb;68(2):579–591. doi: 10.1016/0012-1606(79)90228-8. [DOI] [PubMed] [Google Scholar]
- Janssen G. R., Dworkin M. Cell-cell interactions in developmental lysis of Myxococcus xanthus. Dev Biol. 1985 Nov;112(1):194–202. doi: 10.1016/0012-1606(85)90133-2. [DOI] [PubMed] [Google Scholar]
- Jarvis B. W., Dworkin M. Purification and properties of Myxococcus xanthus cell surface antigen 1604. J Bacteriol. 1989 Sep;171(9):4655–4666. doi: 10.1128/jb.171.9.4655-4666.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis B. W., Dworkin M. Role of Myxococcus xanthus cell surface antigen 1604 in development. J Bacteriol. 1989 Sep;171(9):4667–4673. doi: 10.1128/jb.171.9.4667-4673.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser D. Social gliding is correlated with the presence of pili in Myxococcus xanthus. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5952–5956. doi: 10.1073/pnas.76.11.5952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalos M., Zissler J. Transposon tagging of genes for cell-cell interactions in Myxococcus xanthus. Proc Natl Acad Sci U S A. 1990 Nov;87(21):8316–8320. doi: 10.1073/pnas.87.21.8316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamboj R. K., Siu C. H. Mapping of the monoclonal antibody 80L5C4 epitope on the cell adhesion molecule gp80 of Dictyostelium discoideum. Biochim Biophys Acta. 1988 Nov 10;951(1):78–84. doi: 10.1016/0167-4781(88)90027-9. [DOI] [PubMed] [Google Scholar]
- Kim S. K., Kaiser D. C-factor has distinct aggregation and sporulation thresholds during Myxococcus development. J Bacteriol. 1991 Mar;173(5):1722–1728. doi: 10.1128/jb.173.5.1722-1728.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S. K., Kaiser D. C-factor: a cell-cell signaling protein required for fruiting body morphogenesis of M. xanthus. Cell. 1990 Apr 6;61(1):19–26. doi: 10.1016/0092-8674(90)90211-v. [DOI] [PubMed] [Google Scholar]
- Kim S. K., Kaiser D. Cell motility is required for the transmission of C-factor, an intercellular signal that coordinates fruiting body morphogenesis of Myxococcus xanthus. Genes Dev. 1990 Jun;4(6):896–904. doi: 10.1101/gad.4.6.896. [DOI] [PubMed] [Google Scholar]
- Kroos L., Hartzell P., Stephens K., Kaiser D. A link between cell movement and gene expression argues that motility is required for cell-cell signaling during fruiting body development. Genes Dev. 1988 Dec;2(12A):1677–1685. doi: 10.1101/gad.2.12a.1677. [DOI] [PubMed] [Google Scholar]
- Kroos L., Kaiser D. Expression of many developmentally regulated genes in Myxococcus depends on a sequence of cell interactions. Genes Dev. 1987 Oct;1(8):840–854. doi: 10.1101/gad.1.8.840. [DOI] [PubMed] [Google Scholar]
- Kuner J. M., Kaiser D. Fruiting body morphogenesis in submerged cultures of Myxococcus xanthus. J Bacteriol. 1982 Jul;151(1):458–461. doi: 10.1128/jb.151.1.458-461.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuspa A., Kaiser D. Genes required for developmental signalling in Myxococcus xanthus: three asg loci. J Bacteriol. 1989 May;171(5):2762–2772. doi: 10.1128/jb.171.5.2762-2772.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuspa A., Kroos L., Kaiser D. Intercellular signaling is required for developmental gene expression in Myxococcus xanthus. Dev Biol. 1986 Sep;117(1):267–276. doi: 10.1016/0012-1606(86)90369-6. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lampson L. A., Levy R. Two populations of Ia-like molecules on a human B cell line. J Immunol. 1980 Jul;125(1):293–299. [PubMed] [Google Scholar]
- Leinikki P. O., Calderon J., Luquette M. H., Schreiber R. D. Reduced receptor binding by a human interferon-gamma fragment lacking 11 carboxyl-terminal amino acids. J Immunol. 1987 Nov 15;139(10):3360–3366. [PubMed] [Google Scholar]
- Lindberg F., Lund B., Johansson L., Normark S. Localization of the receptor-binding protein adhesin at the tip of the bacterial pilus. Nature. 1987 Jul 2;328(6125):84–87. doi: 10.1038/328084a0. [DOI] [PubMed] [Google Scholar]
- London J., Allen J. Purification and characterization of a Bacteroides loeschei adhesin that interacts with procaryotic and eucaryotic cells. J Bacteriol. 1990 May;172(5):2527–2534. doi: 10.1128/jb.172.5.2527-2534.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeba P. Y. Iodination of Myxococcus xanthus during development. J Bacteriol. 1983 Sep;155(3):1033–1041. doi: 10.1128/jb.155.3.1033-1041.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthysse A. G. Role of bacterial cellulose fibrils in Agrobacterium tumefaciens infection. J Bacteriol. 1983 May;154(2):906–915. doi: 10.1128/jb.154.2.906-915.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller K., Gerisch G. A specific glycoprotein as the target site of adhesion blocking Fab in aggregating Dictyostelium cells. Nature. 1978 Aug 3;274(5670):445–449. doi: 10.1038/274445a0. [DOI] [PubMed] [Google Scholar]
- Orndorff P. E., Dworkin M. Synthesis of several membrane proteins during developmental aggregation in Myxococcus xanthus. J Bacteriol. 1982 Jan;149(1):29–39. doi: 10.1128/jb.149.1.29-39.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panasenko S. M., Jann B., Jann K. Novel change in the carbohydrate portion of Myxococcus xanthus lipopolysaccharide during development. J Bacteriol. 1989 Apr;171(4):1835–1840. doi: 10.1128/jb.171.4.1835-1840.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panasenko S. M. Methylation of macromolecules during development in Myxococcus xanthus. J Bacteriol. 1985 Nov;164(2):495–500. doi: 10.1128/jb.164.2.495-500.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg E., Keller K. H., Dworkin M. Cell density-dependent growth of Myxococcus xanthus on casein. J Bacteriol. 1977 Feb;129(2):770–777. doi: 10.1128/jb.129.2.770-777.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbluh A., Rosenberg E. Sporulation of Myxococcus xanthus in liquid shake flask cultures. J Bacteriol. 1989 Aug;171(8):4521–4524. doi: 10.1128/jb.171.8.4521-4524.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimkets L. J., Asher S. J. Use of recombination techniques to examine the structure of the csg locus of Myxococcus xanthus. Mol Gen Genet. 1988 Jan;211(1):63–71. doi: 10.1007/BF00338394. [DOI] [PubMed] [Google Scholar]
- Shimkets L. J. Correlation of energy-dependent cell cohesion with social motility in Myxococcus xanthus. J Bacteriol. 1986 Jun;166(3):837–841. doi: 10.1128/jb.166.3.837-841.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimkets L. J., Kaiser D. Induction of coordinated movement of Myxococcus xanthus cells. J Bacteriol. 1982 Oct;152(1):451–461. doi: 10.1128/jb.152.1.451-461.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimkets L. J., Rafiee H. CsgA, an extracellular protein essential for Myxococcus xanthus development. J Bacteriol. 1990 Sep;172(9):5299–5306. doi: 10.1128/jb.172.9.5299-5306.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimkets L. J. Role of cell cohesion in Myxococcus xanthus fruiting body formation. J Bacteriol. 1986 Jun;166(3):842–848. doi: 10.1128/jb.166.3.842-848.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimkets L. J. Social and developmental biology of the myxobacteria. Microbiol Rev. 1990 Dec;54(4):473–501. doi: 10.1128/mr.54.4.473-501.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit G., Kijne J. W., Lugtenberg B. J. Correlation between extracellular fibrils and attachment of Rhizobium leguminosarum to pea root hair tips. J Bacteriol. 1986 Nov;168(2):821–827. doi: 10.1128/jb.168.2.821-827.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit G., Kijne J. W., Lugtenberg B. J. Involvement of both cellulose fibrils and a Ca2+-dependent adhesin in the attachment of Rhizobium leguminosarum to pea root hair tips. J Bacteriol. 1987 Sep;169(9):4294–4301. doi: 10.1128/jb.169.9.4294-4301.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland I. W. Novel surface polymer changes in development of Myxococcus spp. Nature. 1976 Jan 1;259(5538):46–47. doi: 10.1038/259046a0. [DOI] [PubMed] [Google Scholar]
- Tempro P., Cassels F., Siraganian R., Hand A. R., London J. Use of adhesin-specific monoclonal antibodies to identify and localize an adhesin on the surface of Capnocytophaga gingivalis DR2001. Infect Immun. 1989 Nov;57(11):3418–3424. doi: 10.1128/iai.57.11.3418-3424.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanner G., Formanek H., Galli D., Wirth R. Localization of aggregation substances of Enterococcus faecalis after induction by sex pheromones. An ultrastructural comparison using immuno labelling, transmission and high resolution scanning electron microscopic techniques. Arch Microbiol. 1989;151(6):491–497. doi: 10.1007/BF00454864. [DOI] [PubMed] [Google Scholar]
- Weinberg R. A., Zusman D. R. Alkaline, acid, and neutral phosphatase activities are induced during development in Myxococcus xanthus. J Bacteriol. 1990 May;172(5):2294–2302. doi: 10.1128/jb.172.5.2294-2302.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss E. I., London J., Kolenbrander P. E., Andersen R. N., Fischler C., Siraganian R. P. Characterization of monoclonal antibodies to fimbria-associated adhesins of Bacteroides loescheii PK1295. Infect Immun. 1988 Jan;56(1):219–224. doi: 10.1128/iai.56.1.219-224.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss E. I., London J., Kolenbrander P. E., Hand A. R., Siraganian R. Localization and enumeration of fimbria-associated adhesins of Bacteroides loescheii. J Bacteriol. 1988 Mar;170(3):1123–1128. doi: 10.1128/jb.170.3.1123-1128.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson J. R. Exercise and the failing heart. Cardiol Clin. 1987 May;5(2):171–181. [PubMed] [Google Scholar]