Abstract
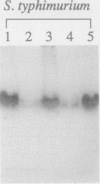
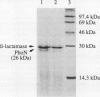
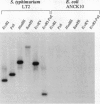
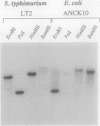
The phoN gene of Salmonella typhimurium encodes nonspecific acid phosphatase (EC 3.1.3.2), which is regulated by a two-component regulatory system consisting of the phoP and phoQ genes. We cloned the phoN region into a plasmid vector by complementation of a phoN mutant strain and determined the nucleotide sequence of the phoN gene and its flanking regions. The phoN gene could encode a 26-kDa protein, which was identified by the maxicell method as the product of phoN. Results of the enzyme assay and Southern hybridization with chromosomal DNA of Escherichia coli K-12 suggests that there is no phoN gene in E. coli. The regulatory pattern of phoN in E. coli and Southern hybridization analysis of the E. coli chromosome with the S. typhimurium phoP gene suggest that E. coli K-12 also harbors the phoP and phoQ genes.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amemura M., Makino K., Shinagawa H., Kobayashi A., Nakata A. Nucleotide sequence of the genes involved in phosphate transport and regulation of the phosphate regulon in Escherichia coli. J Mol Biol. 1985 Jul 20;184(2):241–250. doi: 10.1016/0022-2836(85)90377-8. [DOI] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Chang C. N., Inouye H., Model P., Beckwith J. Processing of alkaline phosphatase precursor to the mature enzyme by an Escherichia coli inner membrane preparation. J Bacteriol. 1980 May;142(2):726–728. doi: 10.1128/jb.142.2.726-728.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C. N., Kuang W. J., Chen E. Y. Nucleotide sequence of the alkaline phosphatase gene of Escherichia coli. Gene. 1986;44(1):121–125. doi: 10.1016/0378-1119(86)90050-8. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Groisman E. A., Chiao E., Lipps C. J., Heffron F. Salmonella typhimurium phoP virulence gene is a transcriptional regulator. Proc Natl Acad Sci U S A. 1989 Sep;86(18):7077–7081. doi: 10.1073/pnas.86.18.7077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henikoff S. Unidirectional digestion with exonuclease III creates targeted breakpoints for DNA sequencing. Gene. 1984 Jun;28(3):351–359. doi: 10.1016/0378-1119(84)90153-7. [DOI] [PubMed] [Google Scholar]
- Inouye H., Beckwith J. Synthesis and processing of an Escherichia coli alkaline phosphatase precursor in vitro. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1440–1444. doi: 10.1073/pnas.74.4.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki H., Shiba T., Nakata A., Shinagawa H. Involvement in DNA repair of the ruvA gene of Escherichia coli. Mol Gen Genet. 1989 Oct;219(1-2):328–331. doi: 10.1007/BF00261196. [DOI] [PubMed] [Google Scholar]
- Kier L. D., Weppelman R. M., Ames B. N. Regulation of nonspecific acid phosphatase in Salmonella: phoN and phoP genes. J Bacteriol. 1979 Apr;138(1):155–161. doi: 10.1128/jb.138.1.155-161.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kier L. D., Weppelman R., Ames B. N. Regulation of two phosphatases and a cyclic phosphodiesterase of Salmonella typhimurium. J Bacteriol. 1977 Apr;130(1):420–428. doi: 10.1128/jb.130.1.420-428.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kier L. D., Weppelman R., Ames B. N. Resolution and purification of three periplasmic phosphatases of Salmonella typhimurium. J Bacteriol. 1977 Apr;130(1):399–410. doi: 10.1128/jb.130.1.399-410.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makino K., Shinagawa H., Amemura M., Kimura S., Nakata A., Ishihama A. Regulation of the phosphate regulon of Escherichia coli. Activation of pstS transcription by PhoB protein in vitro. J Mol Biol. 1988 Sep 5;203(1):85–95. doi: 10.1016/0022-2836(88)90093-9. [DOI] [PubMed] [Google Scholar]
- Makino K., Shinagawa H., Nakata A. Cloning and characterization of the alkaline phosphatase positive regulatory gene (phoM) of Escherichia coli. Mol Gen Genet. 1984;195(3):381–390. doi: 10.1007/BF00341438. [DOI] [PubMed] [Google Scholar]
- Messing J., Crea R., Seeburg P. H. A system for shotgun DNA sequencing. Nucleic Acids Res. 1981 Jan 24;9(2):309–321. doi: 10.1093/nar/9.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller S. I., Kukral A. M., Mekalanos J. J. A two-component regulatory system (phoP phoQ) controls Salmonella typhimurium virulence. Proc Natl Acad Sci U S A. 1989 Jul;86(13):5054–5058. doi: 10.1073/pnas.86.13.5054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakata A., Shinagawa H., Shima H. Alkaline phosphatase isozyme conversion by cell-free extract of Escherichia coli. FEBS Lett. 1984 Oct 1;175(2):343–348. doi: 10.1016/0014-5793(84)80765-6. [DOI] [PubMed] [Google Scholar]
- Pulkkinen W. S., Miller S. I. A Salmonella typhimurium virulence protein is similar to a Yersinia enterocolitica invasion protein and a bacteriophage lambda outer membrane protein. J Bacteriol. 1991 Jan;173(1):86–93. doi: 10.1128/jb.173.1.86-93.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancar A., Wharton R. P., Seltzer S., Kacinski B. M., Clarke N. D., Rupp W. D. Identification of the uvrA gene product. J Mol Biol. 1981 May 5;148(1):45–62. doi: 10.1016/0022-2836(81)90234-5. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons R. W., Houman F., Kleckner N. Improved single and multicopy lac-based cloning vectors for protein and operon fusions. Gene. 1987;53(1):85–96. doi: 10.1016/0378-1119(87)90095-3. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Yamada M., Makino K., Amemura M., Shinagawa H., Nakata A. Regulation of the phosphate regulon of Escherichia coli: analysis of mutant phoB and phoR genes causing different phenotypes. J Bacteriol. 1989 Oct;171(10):5601–5606. doi: 10.1128/jb.171.10.5601-5606.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]