Abstract
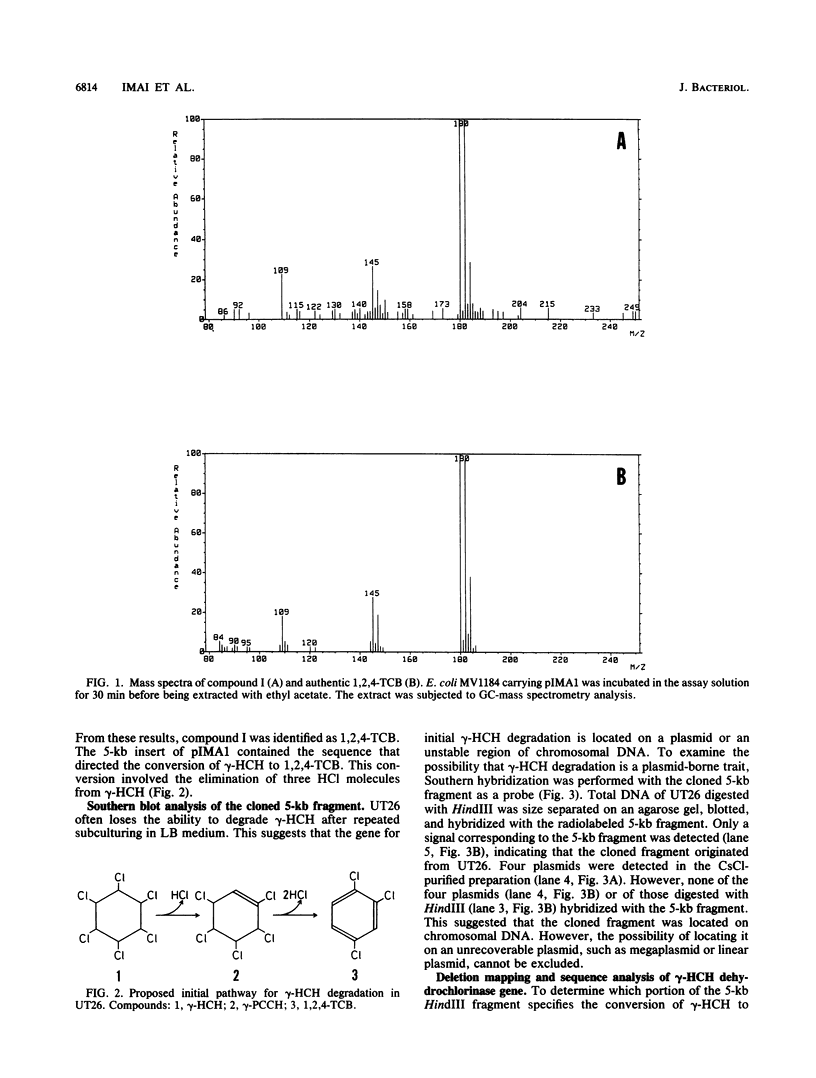
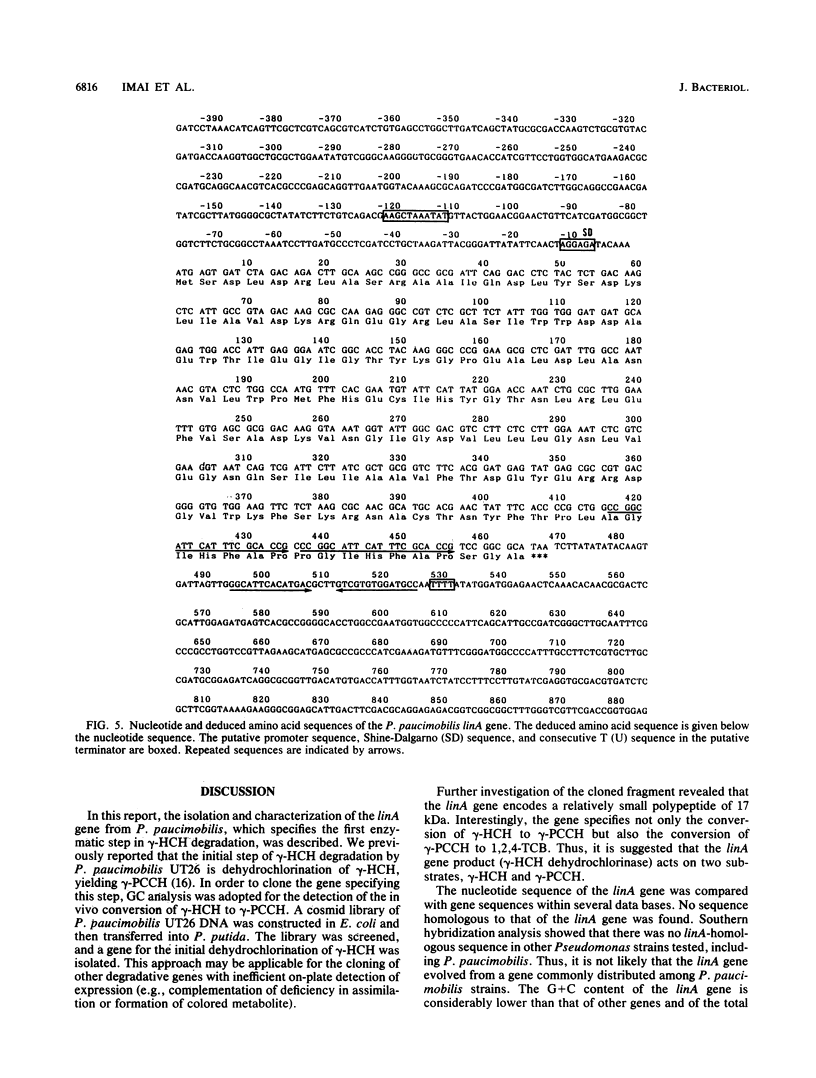
Pseudomonas paucimobilis UT26 is capable of growing on gamma-hexachlorocyclohexane (gamma-HCH). A genomic library of P. paucimobilis UT26 was constructed in Pseudomonas putida by using the broad-host-range cosmid vector pKS13. After 2,300 clones were screened by gas chromatography, 3 clones showing gamma-HCH degradation were detected. A 5-kb fragment from one of the cosmid clones was subcloned into pUC118, and subsequent deletion and gas chromatography-mass spectrometry analyses revealed that a fragment of ca. 500 bp was responsible for the conversion of gamma-HCH to 1,2,4-trichlorobenzene via gamma-pentachlorocyclohexene. Nucleotide sequence analysis revealed an open reading frame (linA) of 465 bp within the fragment. The nucleotide sequence of the linA gene and the deduced amino acid sequence showed no similarity to any known sequences. The product of the linA gene was 16.5 kDa according to sodium dodecyl sulfate-polyacrylamide gel electrophoresis.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arca P., Hardisson C., Suárez J. E. Purification of a glutathione S-transferase that mediates fosfomycin resistance in bacteria. Antimicrob Agents Chemother. 1990 May;34(5):844–848. doi: 10.1128/aac.34.5.844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann A., Walet P., Wijnen P., de Bruin W., Huntjens J. L., Roelofsen W., Zehnder A. J. Biodegradation of alpha- and beta-hexachlorocyclohexane in a soil slurry under different redox conditions. Appl Environ Microbiol. 1988 Jan;54(1):143–149. doi: 10.1128/aem.54.1.143-149.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnsley E. A. Role and regulation of the ortho and meta pathways of catechol metabolism in pseudomonads metabolizing naphthalene and salicylate. J Bacteriol. 1976 Feb;125(2):404–408. doi: 10.1128/jb.125.2.404-408.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark A. G., Hitchcock M., Smith J. N. Metabolism of gammexane in flies, ticks and locusts. Nature. 1966 Jan 1;209(5018):103–103. doi: 10.1038/209103a0. [DOI] [PubMed] [Google Scholar]
- Don R. H., Weightman A. J., Knackmuss H. J., Timmis K. N. Transposon mutagenesis and cloning analysis of the pathways for degradation of 2,4-dichlorophenoxyacetic acid and 3-chlorobenzoate in Alcaligenes eutrophus JMP134(pJP4). J Bacteriol. 1985 Jan;161(1):85–90. doi: 10.1128/jb.161.1.85-90.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Figurski D. H., Helinski D. R. Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1648–1652. doi: 10.1073/pnas.76.4.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frantz B., Chakrabarty A. M. Organization and nucleotide sequence determination of a gene cluster involved in 3-chlorocatechol degradation. Proc Natl Acad Sci U S A. 1987 Jul;84(13):4460–4464. doi: 10.1073/pnas.84.13.4460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa K., Arimura N., Miyazaki T. Nucleotide sequence of the 2,3-dihydroxybiphenyl dioxygenase gene of Pseudomonas pseudoalcaligenes. J Bacteriol. 1987 Jan;169(1):427–429. doi: 10.1128/jb.169.1.427-429.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa K., Miyazaki T. Cloning of a gene cluster encoding biphenyl and chlorobiphenyl degradation in Pseudomonas pseudoalcaligenes. J Bacteriol. 1986 May;166(2):392–398. doi: 10.1128/jb.166.2.392-398.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heritage A. D., MacRae I. C. Degradation of lindane by cell-free preparations of Clostridium sphenoides. Appl Environ Microbiol. 1977 Aug;34(2):222–224. doi: 10.1128/aem.34.2.222-224.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heritage A. D., Rae I. C. Identification of intermediates formed during the degradation of hexachlorocyclohexanes by Clostridium sphenoides. Appl Environ Microbiol. 1977 Jun;33(6):1295–1297. doi: 10.1128/aem.33.6.1295-1297.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iizuka M., Inoue Y., Murata K., Kimura A. Purification and some properties of glutathione S-transferase from Escherichia coli B. J Bacteriol. 1989 Nov;171(11):6039–6042. doi: 10.1128/jb.171.11.6039-6042.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inouye S., Asai Y., Nakazawa A., Nakazawa T. Nucleotide sequence of a DNA segment promoting transcription in Pseudomonas putida. J Bacteriol. 1986 Jun;166(3):739–745. doi: 10.1128/jb.166.3.739-745.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagnow G., Haider K., Ellwardt P. C. Anaerobic dechlorination and degradation of hexachlorocyclohexane isomers by anaerobic and facultative anaerobic bacteria. Arch Microbiol. 1977 Dec 15;115(3):285–292. doi: 10.1007/BF00446454. [DOI] [PubMed] [Google Scholar]
- Kimbara K., Hashimoto T., Fukuda M., Koana T., Takagi M., Oishi M., Yano K. Cloning and sequencing of two tandem genes involved in degradation of 2,3-dihydroxybiphenyl to benzoic acid in the polychlorinated biphenyl-degrading soil bacterium Pseudomonas sp. strain KKS102. J Bacteriol. 1989 May;171(5):2740–2747. doi: 10.1128/jb.171.5.2740-2747.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kok M., Oldenhuis R., van der Linden M. P., Meulenberg C. H., Kingma J., Witholt B. The Pseudomonas oleovorans alkBAC operon encodes two structurally related rubredoxins and an aldehyde dehydrogenase. J Biol Chem. 1989 Apr 5;264(10):5442–5451. [PubMed] [Google Scholar]
- LIPKE H., KEARNS C. W. DDT dehydrochlorinase. I. Isolation, chemical properties, and spectrophotometric assay. J Biol Chem. 1959 Aug;234(8):2123–2128. [PubMed] [Google Scholar]
- La Roche S. D., Leisinger T. Sequence analysis and expression of the bacterial dichloromethane dehalogenase structural gene, a member of the glutathione S-transferase supergene family. J Bacteriol. 1990 Jan;172(1):164–171. doi: 10.1128/jb.172.1.164-171.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- MacRae I. C., Raghu K., Bautista E. M. Anaerobic degradation of the insecticide lindane by Clostridium sp. Nature. 1969 Mar 1;221(5183):859–860. doi: 10.1038/221859a0. [DOI] [PubMed] [Google Scholar]
- Marks T. S., Allpress J. D., Maule A. Dehalogenation of lindane by a variety of porphyrins and corrins. Appl Environ Microbiol. 1989 May;55(5):1258–1261. doi: 10.1128/aem.55.5.1258-1261.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda Y., Nishikawa S., Shiozuka K., Kadokura H., Nakajima H., Yoda K., Katayama Y., Morohoshi N., Haraguchi T., Yamasaki M. Molecular cloning of the protocatechuate 4,5-dioxygenase genes of Pseudomonas paucimobilis. J Bacteriol. 1990 May;172(5):2704–2709. doi: 10.1128/jb.172.5.2704-2709.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohisa N., Kurihara N., Nakajima M. ATP synthesis associated with the conversion of hexachlorocyclohexane related compounds. Arch Microbiol. 1982 Jun;131(4):330–333. doi: 10.1007/BF00411180. [DOI] [PubMed] [Google Scholar]
- Ohisa N., Yamaguchi M., Kurihara N. Lindane degradation by cell-free extracts of Clostridium rectum. Arch Microbiol. 1980 Apr;125(3):221–225. doi: 10.1007/BF00446880. [DOI] [PubMed] [Google Scholar]
- Sahu S. K., Patnaik K. K., Sharmila M., Sethunathan N. Degradation of Alpha-, Beta-, and Gamma-Hexachlorocyclohexane by a Soil Bacterium under Aerobic Conditions. Appl Environ Microbiol. 1990 Nov;56(11):3620–3622. doi: 10.1128/aem.56.11.3620-3622.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shine J., Dalgarno L. Determinant of cistron specificity in bacterial ribosomes. Nature. 1975 Mar 6;254(5495):34–38. doi: 10.1038/254034a0. [DOI] [PubMed] [Google Scholar]
- Streber W. R., Timmis K. N., Zenk M. H. Analysis, cloning, and high-level expression of 2,4-dichlorophenoxyacetate monooxygenase gene tfdA of Alcaligenes eutrophus JMP134. J Bacteriol. 1987 Jul;169(7):2950–2955. doi: 10.1128/jb.169.7.2950-2955.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taira K., Hayase N., Arimura N., Yamashita S., Miyazaki T., Furukawa K. Cloning and nucleotide sequence of the 2,3-dihydroxybiphenyl dioxygenase gene from the PCB-degrading strain of Pseudomonas paucimobilis Q1. Biochemistry. 1988 May 31;27(11):3990–3996. doi: 10.1021/bi00411a015. [DOI] [PubMed] [Google Scholar]
- Terada I., Kwon S. T., Miyata Y., Matsuzawa H., Ohta T. Unique precursor structure of an extracellular protease, aqualysin I, with NH2- and COOH-terminal pro-sequences and its processing in Escherichia coli. J Biol Chem. 1990 Apr 25;265(12):6576–6581. [PubMed] [Google Scholar]
- Tu C. M. Utilization and degradation of lindane by soil microorganisms. Arch Microbiol. 1976 Jul;108(3):259–263. doi: 10.1007/BF00454850. [DOI] [PubMed] [Google Scholar]
- Vieira J., Messing J. Production of single-stranded plasmid DNA. Methods Enzymol. 1987;153:3–11. doi: 10.1016/0076-6879(87)53044-0. [DOI] [PubMed] [Google Scholar]
- Weisshaar M. P., Franklin F. C., Reineke W. Molecular cloning and expression of the 3-chlorobenzoate-degrading genes from Pseudomonas sp. strain B13. J Bacteriol. 1987 Jan;169(1):394–402. doi: 10.1128/jb.169.1.394-402.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]